Atrovent Nasal Drug / Medicine Information - News-Medical.net
Active ingredient(s): Ipratropium bromide monohydrate
Consumer Medicine Information (CMI)
This leaflet provides important information about using Atrovent Nasal. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Atrovent Nasal.
Where to find information in this leaflet:
Why am I using Atrovent Nasal?
Atrovent Nasal contains the active ingredient ipratropium bromide monohydrate. Atrovent Nasal is an anticholinergic medicine. It belongs to one of several types of medicines used to treat the symptoms of rhinitis (runny nose). Atrovent Nasal relieves a runny nose by acting directly on the mucous glands of the nose to decrease watery secretions.
Atrovent Nasal is used to relieve the symptoms of a persistent runny nose associated with allergic rhinitis (hayfever) and non-allergic non-seasonal rhinitis.
What should I know before I use Atrovent Nasal?
Warnings
Do not use Atrovent Nasal if:
1. you are allergic to ipratropium bromide, or any of the ingredients listed at the end of this leaflet. Always check the ingredients to make sure you can use this medicine.
2. you are allergic to any other medicine, particularly atropine or other anticholinergic medicines.
3. the packaging is torn or shows signs of tampering.
4. it is past the expiry date printed on the pack.
Check with your doctor or pharmacist if you:
have any other medical conditions, especially:
an eye problem called "glaucoma"
enlargement of the prostate gland
problems with passing urine
cystic fibrosis
are uncertain as to whether you have, or have had, any of these conditions.
take any medicines for any other condition.
Atrovent Nasal spray contains the (antimicrobial) preservative benzalkonium chloride which may cause irritation of the nasal lining (mucosa).
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. Care is recommended during pregnancy, particularly in the first three months.
Talk to your doctor if you are breastfeeding or intend to breastfeed. Care is also recommended if you are breastfeeding as it is not known if the active ingredient in Atrovent Nasal may pass into breast milk.
The benefits of Atrovent Nasal must be assessed against any risks. Your doctor will discuss the risks and benefits of using Atrovent Nasal while you are pregnant or breastfeeding.
Children
There is not enough information available to recommend the use of Atrovent Nasal in children under 12 years of age.
What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
In particular, you should tell your doctor if you are taking any other medicine containing ipratropium bromide or another anticholinergic agent such as atropine, hyoscine or hyoscyamine.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Atrovent Nasal.
How do I use Atrovent Nasal?
How much to use
The usual dose for adults and children 12 years of age and over is 2 to 4 sprays into each nostril two or three times daily (at regular intervals).
If your doctor or pharmacist has changed this dose you should ask for further information from your doctor or pharmacist.
Do not use extra doses of Atrovent Nasal without consulting your doctor or pharmacist.
When to use Atrovent Nasal
Atrovent Nasal should be used two or three times daily (at regular intervals).
If you are not sure when to use it, ask your doctor or pharmacist.
How to use Atrovent Nasal
Important Information:
Read complete directions carefully and use only as directed.
Do not spray in or around your eyes.
Do not pierce the nozzle or attempt to enlarge the hole. The hole in the nozzle is a special size to allow the correct dose of Atrovent Nasal to enter your nostril when you depress the spray pump.
Do not shake the bottle.
To Use:
1. Remove protective cap.
2. Before using the spray pump for the first time, rapidly activate the spray pump 5 to 7 times until an even spray mist is released (see Figure 1). Your Atrovent Nasal is now primed and ready for use.
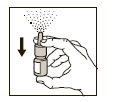
(Figure 1)
With subsequent use, the spray pump is immediately functional. However, if the spray pump has not been used for more than 24 hours, it will require repriming by performing step 2 above.
2. Blow nose thoroughly before using the nasal spray.
3. Keeping the bottle upright, insert the spray adaptor into the nostril and activate the spray pump firmly while breathing in gently through the nose (see Figure 2).
Administer the number of sprays as recommended or as prescribed by your doctor or pharmacist and then repeat in the other nostril.
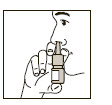
(Figure 2)
5. Replace protective cap after use.
6. Store upright
7. To Clean:
If the nasal tip becomes clogged, remove protective cap. Hold the nasal tip under running, warm tap water (see Figure 3) for about a minute. Dry the nasal tip, reprime the nasal pump spray (step 2 above), and replace the protective cap.
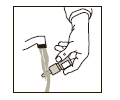
(Figure 3)
If you forget to use Atrovent Nasal
Atrovent Nasal should be used as directed. If you miss your dose at the usual time, use it as soon as you remember.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much Atrovent Nasal
If you think that you have used too much Atrovent Nasal, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling 13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Signs of overdose may include increased heart rate, dry mouth and blurred vision.
What should I know while using Atrovent Nasal?
Things you should do
Follow the Directions For Use carefully and avoid contact with the eyes. If the spray from Atrovent Nasal comes into direct contact with the eyes, it may cause eye pain, blurring of vision and increased sensitivity to light.
It may also cause a more serious eye problem, known as glaucoma, to develop or worsen. The following symptoms may be associated with glaucoma: red eyes, eye pain or discomfort, sight disturbances (blurred vision, visual halos or coloured images).
Tell any other doctors, dentists and pharmacists who are treating you that you are using Atrovent Nasal.
If you are about to be started on any new medicine, tell your doctor or pharmacist that you are using Atrovent Nasal.
If you become pregnant, while using Atrovent Nasal, tell your doctor or pharmacist.
Call your doctor straight away if you:
experience eye pain or discomfort, or any disturbances with your sight (blurred vision, visual halos or coloured images) during or after using Atrovent Nasal.
Remind any doctor or dentist you visit that you are using Atrovent Nasal.
Things you should not do
Never spray Atrovent Nasal in or around your eyes.
If you accidentally spray Atrovent Nasal into your eyes, you may have temporary blurring of vision and increased sensitivity to light, lasting up to a few hours.
Contact your doctor immediately for advice as to what treatment is needed.
Do not use Atrovent Nasal when the medicine is running out and it is no longer possible to obtain a firm spray.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Atrovent Nasal affects you.
Atrovent Nasal may cause dizziness in some people
If side effects are experienced such as dizziness, blurred vision or dilated pupils (mydriasis) whilst using Atrovent Nasal do not drive or operate machinery.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
Atrovent Nasal should be kept in a cool, dry place where the temperature stays below 25°C.
Do not store in direct sunlight or heat and protect from freezing.
Follow the instructions in the carton on how to take care of your medicine properly.
It is recommended that the spray adaptor be cleaned after use.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
Discard the medicine if it has been 12 months after first opening or if the expiry date has passed.
Getting rid of any unwanted medicine
If you no longer need to use Atrovent Nasal anymore or it is out of date, take it to any pharmacy for safe disposal.
Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
Product details
What Atrovent Nasal contains
| Active ingredient (main ingredient) | 22 micrograms (µg) ipratropium bromide monohydrate per spray [equivalent to ipratropium bromide 21 micrograms] |
|---|---|
| Other ingredients (inactive ingredients) | benzalkonium chloride as a preservative disodium edetate sodium chloride (salt) sodium hydroxide hydrochloric acid |
Do not use this medicine if you are allergic to any of these ingredients.
What Atrovent Nasal looks like
Atrovent Nasal is a clear, colourless solution in amber glass bottles. Each bottle contains 15 mL of solution which provides at least 180 metered doses. (Aust R 53362).
Who distributes Atrovent Nasal
Distributed in Australia by:
Sanofi Consumer Healthcare,
87 Yarraman Place, Virginia,
Qld 4014 Australia.
Toll-free: 1800 818 806
This leaflet was prepared in February 2021.
atro-ccds0261-00-cmiv7-feb21

Comments
Post a Comment