COVID-19-associated fungal infections - Nature.com
Abstract
Coronavirus disease 2019 (COVID-19)-associated invasive fungal infections are an important complication in a substantial number of critically ill, hospitalized patients with COVID-19. Three groups of fungal pathogens cause co-infections in COVID-19: Aspergillus, Mucorales and Candida species, including Candida auris. Here we review the incidence of COVID-19-associated invasive fungal infections caused by these fungi in low-, middle- and high-income countries. By evaluating the epidemiology, clinical risk factors, predisposing features of the host environment and immunological mechanisms that underlie the pathogenesis of these co-infections, we set the scene for future research and development of clinical guidance.
Main
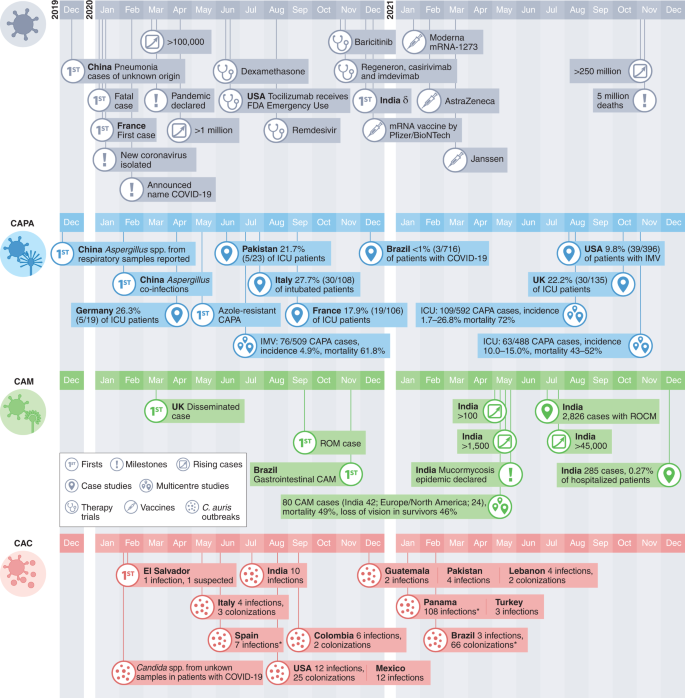
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has rapidly spread across the globe, accounting for almost 300 million cases of coronavirus disease 2019 (COVID-19) so far (Fig. 1). The first cases of COVID-19-associated pulmonary aspergillosis (CAPA) were reported from China in early 20201 (Fig. 1). Since then, multiple case series and cohort studies have highlighted the importance of this potentially life-threatening secondary infection, sometimes caused by azole-resistant Aspergillus spp.2. The most commonly affected patients are those with acute respiratory failure due to COVID-19, particularly patients receiving systemic corticosteroids or tocilizumab therapies3,4,5,6,7,8,9,10. The outcomes of CAPA have been devastating, with reports on the independent contribution of CAPA estimating mortality rates of more than 40% (refs. 4,6,9,11) (Fig. 2).
*Not specified if in patients with COVID-19/unclear reported numbers. IMV, invasive mechanical ventilation.
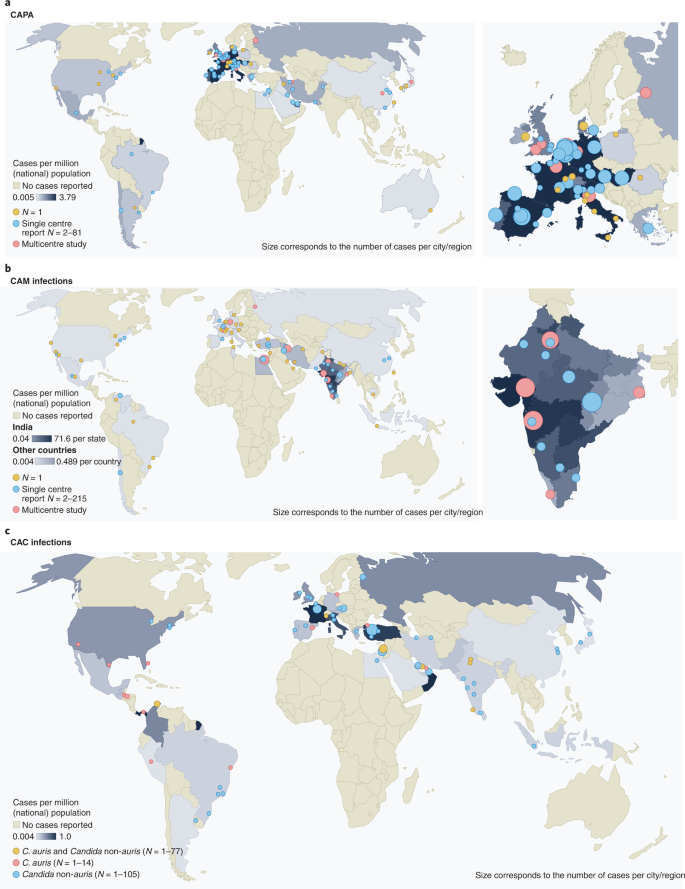
The CAPA (a), CAM (b) and CAC (c) distributions, including C. auris infections for countries that reported respective cases. References can be found in Supplementary Table 1. a,b, The dots represent cities where cases occurred (yellow, single case; blue, at least two cases at one site; red, multicentre studies/multiple sites per city/national reports). The colour of countries and the size of the dots are proportional to the number of cases per million population of the respective country. c, The dots represent cities where cases occurred (red, C. auris; yellow, C. auris and Candida non-auris cases; blue, Candida non-auris cases). The colour of countries and size of the dots are proportional to the number of cases per million population of the respective country (cut-off at 10 million population: El Salvador, Lebanon, Panama, Qatar and United Arab Emirates).
COVID-19-associated mucormycosis (CAM) gained worldwide attention in early 2021, during the second wave of the COVID-19 pandemic in India (Fig. 1). An unprecedented surge of cases of mucormycosis, a fungal infection caused by moulds belonging to the order Mucorales, posed a major healthcare problem with >47,500 cases reported by the Indian government between May and August 2021 (https://governmentstats.com/mucormycosis/index.html). This was covered in the lay press wrongly as a 'black fungus' pandemic, referring to the colour of the necrotic tissue as a result of the destructive nature of the fungal pathogen, but failing to consider that the term black fungus actually refers to a different category of fungal pathogens that is not associated with CAM12,13 (Fig. 2 and Supplementary Table 1). Uncontrolled diabetes, and systemic corticosteroid (over)treatment, are two risk factors for the development of CAM in India and other countries14. While disease manifestation and incidence vary considerably between different geographical regions15, rhino-orbital-cerebral and pulmonary diseases are the most frequent clinical presentations of CAM in patients with COVID-19. Mucormycosis adds particular insult to injury in a substantial proportion of critically ill patients with COVID-19 due to high mortality rates and because this disease is necrotizing and often requires aggressive surgical debridement, which results in disfigurement in those who survive16.
Invasive Candida infections in patients with COVID-19 in the intensive care unit (ICU) were first described shortly after the emergence of SARS-CoV-217 (Fig. 1). Common risk factors in patients in the ICU include the use of antibiotics, central venous catheters and corticosteroids; invasive Candida infections impose considerable comorbidity. Of particular concern are outbreaks of severe Candida auris infections in patients with COVID-19 in the ICU18,19 (Fig. 2). C. auris is difficult to distinguish from other Candida species and is often resistant to many or all antifungal drugs20. It can persist on surfaces; unlike other invasive fungal infections (IFIs), it can be transmitted in healthcare facilities.
SARS-CoV-2 infection alters immune and metabolic responses in patients, which together produce an inflammatory environment that is highly permissive to fungal infections; however, the underlying mechanisms are complex. In this Review, we discuss the epidemiology, risk factors, predisposing features of the host environment and immunological mechanisms that are involved in the pathogenesis of COVID-19 fungal co-infections.
Epidemiology and risk factors
CAPA
Cases of CAPA emerged during the first months of the pandemic. However, incidence rates varied widely, probably because CAPA is difficult to diagnose in patients with COVID-19-associated acute respiratory distress syndrome (ARDS). Specifically, clinical features and radiological findings of CAPA resemble those of severe COVID-192 and blood tests lack sensitivity6,21,22,23 due to the invasive growth of Aspergillus in the airway and the clearance of Aspergillus galactomannan (GM) from systemic circulation by neutrophils in non-neutropenic patients24,25,26. This results in a clinical picture of CAPA that is different from primarily angioinvasive invasive pulmonary aspergillosis (IPA) observed in patients with neutropenia; CAPA is often limited to airway invasive growth for several days before it becomes angioinvasive27. However, once CAPA becomes angioinvasive and produces positive serum GM, mortality is more than 80%, even if systemic antifungal therapy is provided28,29. Testing of bronchoalveolar lavage (BAL) for CAPA enables earlier detection21,22,23, but due to the risk of COVID-19 transmission, bronchoscopies were rarely performed, particularly during the early phase of the pandemic30. Lack of autopsies may also have resulted in an underestimation of CAPA prevalence in some hospitals31,32.
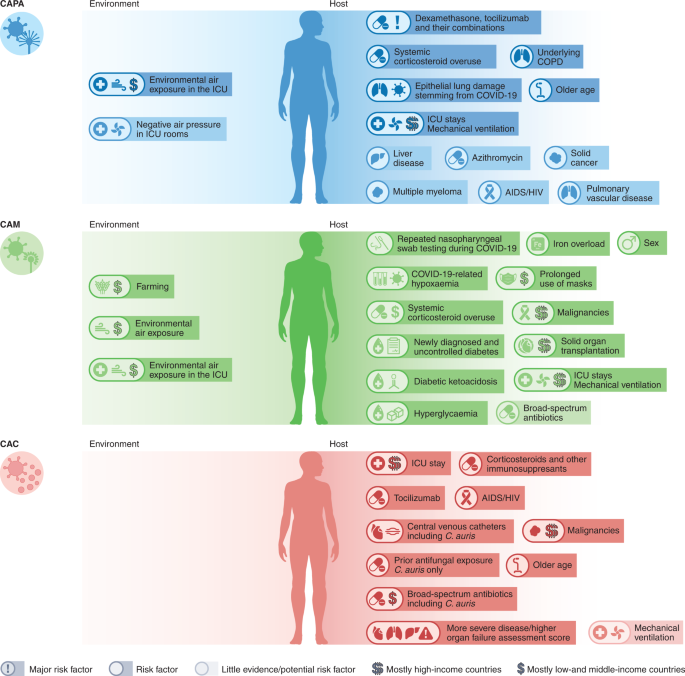
In the absence of consensus definitions for CAPA, classification criteria were modified33,34; these modifications varied widely between studies and often included non-specific mycological evidence such as culture results or even GM testing of tracheal aspirates. Using these variable criteria, a median incidence of 20.1% (range 1.6–38%) was reported in patients with COVID-19 acute respiratory failure requiring invasive ventilation35,36,37,38. Implementation of more conservative European Confederation of Medical Mycology/International Society for Human and Animal Mycoses consensus criteria to detect CAPA in late 2020 (requires culture, GM enzyme-linked immunosorbent assay, lateral flow assay or Aspergillus PCR from either BAL or blood for classification of probable CAPA)25 together with non-bronchoscopic lavage for possible CAPA, has resulted in a substantial reduction of the mean incidence of probable/proven CAPA cases4,7,31, bringing the prevalence of CAPA down to about 10% among invasively ventilated patients with COVID-19. However, incidence continues to vary widely between ICUs, due to non-uniform approaches to COVID-19 treatments, different burdens of Aspergillus exposure and differing diagnostic algorithms as well as genetic predisposing risk factors39,40. The combination of dexamethasone and tocilizumab, invasive ventilation and older age, have been reported as risk factors for developing CAPA4 (Table 1, Fig. 3 and Supplementary Table 2). Unlike influenza-associated pulmonary aspergillosis, CAPA develops later and is diagnosed a median of 8 days after ICU admission4,6,7. CAPA has been consistently associated with COVID-19 mortality rates of more than 50% (ref. 6,7,8,9); the combination of Aspergillus growth from BAL fluid culture and positive BAL fluid GM can predict 90-day mortality (hazard ratio 2.53), even in the absence of a positive serum GM41. In the largest multicentre studies, CAPA is an independent prognostic factor for mortality, with hazard ratios between 1.45 and 1.97 (refs. 4,11).
References can be found in Supplementary Table 2.
COVID-19-associated mucormycosis
Before the COVID-19 pandemic, India and Pakistan had the highest burden of invasive mucormycosis, predominantly occurring in men42. Indian population-based case rates of mucormycosis estimate the burden of CAM to be 70-times higher than the global rate, with predicted annual cases of >200,000 before the pandemic43,44. The high incidence of CAM has been linked to environmental factors, such as exposure to fungal spores43,45 and chronic illness, such as poorly controlled diabetes mellitus46. In high-income countries, common underlying conditions are haematological malignancies and organ transplants. Further predisposing factors include use of immunosuppressants, such as corticosteroids, and neutropenia (Table 1 and Fig. 3)45.
These known risk factors, combined with the changes in metabolism and immune function owing to COVID-19 and COVID-19 treatment modalities, resulted in a surge of CAM cases particularly in low- and middle-income countries. While CAM cases in patients with classical risk factors, such as underlying haematological malignancies, occurred during the first months of the pandemic, the number of reported cases of CAM increased dramatically in the second wave of the pandemic, possibly driven by the use (sometimes overuse) of systemic corticosteroids, by the increasing case numbers in low- and middle-income countries with high environmental Mucorales exposure and by an increase in patients with uncontrolled or undiagnosed diabetes. Globally, the highest number of cases was reported from India, with such dramatic increases compared with pre-COVID-19 rates that the Central Government of India declared a mucormycosis epidemic on 10 May 2021. Prevalence of CAM in India was 0.27% in hospitalized patients with COVID-19 and 1.6% in patients managed in the ICU15 (Fig. 2). Estimates on incidence rates in other countries are scarce, although CAM cases have been described in countries in Asia, Africa, Europe and South, Middle and North America14,47 (Fig. 2).
In contrast to the large case series from India, with patients suffering from either rhino-orbital mucormycosis (ROM) or rhino-orbital cerebral mucormycosis (ROCM), which have a distinct clinical presentation that is rarely missed, in high-income countries the site of involvement is primarily pulmonary or disseminated. Lack of diagnosis of CAM might arise because the clinical and radiological presentation of pulmonary and disseminated mucormycosis overlaps with the clinical features of COVID-1916,48. In fact, CAM is sometimes only diagnosed if invasive diagnostics, including histology and autopsies, are used48. Together this means that it is likely that the true burden of CAM in high-income countries is underestimated. In addition, the geographical distribution of caseloads with independent risk factors for ROM/ROCM, such as diabetes mellitus, may contribute to the observed epidemiology of COVID-19-associated CAM46.
Rhizopus spp. are the predominant pathogen causing CAM in case series from India and other countries14,15,47,49. Most ROM/ROCM CAM cases are diagnosed about 10 days after SARS-CoV-2 detection; however, delayed diagnosis up to three months post-COVID-19 has been reported14,49. Depending on the clinical manifestation, mortality rates differ14. COVID-19-associated ROM has mortality rates of 14% and higher49, whereas pulmonary or disseminated mucormycosis has mortality rates in excess of 80%14.
COVID-19-associated candidiasis including C. auris
A substantial proportion of patients with COVID-19 require ICU treatment, parenteral nutrition and mechanical ventilation, which together with systemic corticosteroid therapy, predisposes them to infections with Candida spp. In some studies, a two- to tenfold higher incidence of candidemia has been reported in patients with COVID-19 compared with patients without COVID-1950,51. Candida albicans is the most frequently reported yeast species in critically ill patients with COVID-19 (44% of candidemia cases in one US multicentre study52) but in some geographical areas the emerging pathogen C. auris was predominant53.
Since C. auris was first identified in Japan in 2009, case numbers of infection have increased globally. It can colonize the skin and environmental surfaces and can be transmitted as a nosocomial pathogen54. These features have made C. auris a global fungal public health threat. Infection with C. auris and other Candida species is associated with prolonged stay in the ICU, administration of broad-spectrum antibiotics and systemic steroids, central lines, diabetes mellitus, kidney disease and mechanical ventilation (Fig. 3 and Table 1)55,56. Prior exposure to antifungals is a major risk factor for C. auris bloodstream infection57. Tocilizumab use has also been linked to C. auris fungemia in patients with COVID-1958,59.
Under-resourced public health systems, increased workload of healthcare workers and over-occupancy of beds during the COVID-19 pandemic are negatively impacting infection control measures60. The first reported cases of C. auris bloodstream infections in patients with COVID-19 were in February 202061. Since then, 13 outbreaks have been reported worldwide, ranging from 2 to 12 infections each and multiple colonized patients in addition (Figs. 1 and 2). Commercially available biochemical-based tests may misidentify C. auris, making management in resource-limited countries another challenge. Consequently, the true scale of infections may be underestimated. Currently, no reliable data on incidence in patients with COVID-19 globally can be extrapolated. COVID-19-associated C. auris outbreaks have resulted in mortality rates ranging from 30% (ref. 62) to 83% (ref. 19) in those with candidemia. Corticosteroid use, sepsis and older age have been identified as independent risk factors for mortality in patients with COVID-19-associated candidiasis (CAC)50.
Pathogenesis of COVID-19-associated IFIs
COVID-19-associated immune dysfunction
The immunopathogenesis of COVID-19-associated IFIs is not completely understood but involves multiple factors caused by the viral infection, fungus and host immune response63. SARS-CoV-2 targets cells expressing angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), including airway epithelial cells, type 2 pneumocytes, vascular endothelial cells and alveolar macrophages64. The infection causes airway epithelial damage, characterized by disrupted epithelial junctions, impaired ciliary clearance and functional defects, such as the release of antimicrobial proteins65. During fungal infection, airway fibrinogenolysis and disruption of epithelial tight junctions by fungal proteases during germination, such as alkaline protease 1 from Aspergillus spp., can promote allergic inflammation and further sustain lung pathology66. The obstruction of the airways due to the release of fibrinous material from the tissue and dying cells decrease oxygen and carbon dioxide diffusion; this hypoxic milieu can influence both fungal virulence and the host immune response67. This results in a vicious circle, in which secondary fungal metabolites such as gliotoxin and mucoricin can further increase both local and systemic hypoxia by damaging host tissues, restraining angiogenesis and tissue repair68,69.
On viral infection, injured epithelial cells may also express apical receptors, such as integrins70, which in turn mediate the interaction with proteins on the surface of the Aspergillus, Mucorales or Candida cell wall, namely the thaumatin-like protein CalA, spore-coating (CotH) proteins and mannoproteins, respectively, to promote fungal adherence and invasion71,72,73. Fungal adhesion can be further enhanced by the expression of fungal cell wall components, such as galactosaminogalactan in Aspergillus, which also mediates biofilm formation74. On the other hand, the influenza virus can hijack the cytoskeletal system to allow viral entry and replication75, a feature that may, to an extent, also facilitate fungal invasion. In this regard, gliotoxin can promote actin cytoskeleton rearrangements in epithelial cells to favour conidia internalization76. Moreover, gliotoxin and other secondary metabolites, such as fumagillin, also contribute to immune evasion by inhibiting immune cell function77.
Virus-infected cells release danger-associated molecular patterns (DAMPs), which act as danger signals on adjacent epithelial cells and resident alveolar macrophages to promote the release of proinflammatory cytokines and chemokines, and elicit the influx of macrophages and neutrophils, exacerbating the local inflammatory response78. DAMPs have been implicated in the regulation of inflammation in fungal diseases79; in particular, the DAMP/receptor for advanced glycation end products (AGEs) axis was found to integrate signals derived from Toll-like receptors (TLRs) and amplify inflammatory responses in experimental aspergillosis79. In this regard, genetic variants causing the hyperactivation of danger signalling were identified as risk factors for the development of invasive aspergillosis in immunocompromised hosts80. Chronic hyperglycaemia also promotes the irreversible glycation and oxidation of proteins and lipids leading to the formation of AGEs81 and these could be presumably implicated in the pathogenesis of mucormycosis.
Hyperinflammation is a hallmark of COVID-19, characterized by high levels of circulating proinflammatory cytokines, including interleukin-6 (IL-6), interferon-γ (IFN-γ), IL-1β and tumour necrosis factor (TNF), and acute phase reactants and ferritin82 (Fig. 4). The activation of antiviral immunity after viral recognition by innate immune cells might also paradoxically promote systemic inflammatory reactions and establish a highly permissive environment to the development of fungal co-infections by potentiating the expression of specific virulence factors and damage to the host. While these cytokines are essential for the innate control of fungal infection, an exacerbated production instead disrupts immune homeostasis and drives a pathogenic activation of the immune system that leads to tissue damage and fungal invasion83. In this regard, single-cell RNA sequencing and proteomics applied to blood samples from patients with severe COVID-19 have revealed defective monocyte activation and dysregulated myelopoiesis with release of immature dysfunctional neutrophils and monocytes into the circulation84. High-dimensional flow cytometry analyses also revealed an expansion of intermediate monocytes that exhibit a proinflammatory phenotype85,86.
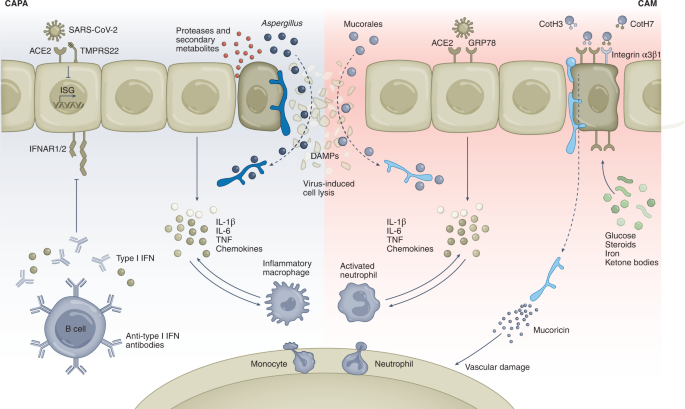
SARS-CoV-2 infects alveolar epithelial cells by binding to the ACE2 receptor with the help of TMPRSS2. The virus represses type I IFN responses by blocking the transcription of IFN-stimulated genes and pre-existing genetic variants; type I IFN autoantibodies add further to this effect, preventing signalling through the IFNAR1/2 receptor complex. Delayed type I IFN responses favour the release of proinflammatory cytokines and chemokines, leading to the recruitment of monocyte-derived macrophages and neutrophils into the lungs that further contribute to cytokine and chemokine production. This general dysregulation of the immune response underlies a hyperinflammatory state that results in a 'cytokine storm' and ARDS that ultimately favours the development of fungal co-infections. During COVID-19, virus-induced cell lysis favours the invasion of the tissue by fungi; the release of DAMPs from damaged or dying cells further potentiates the hyperinflammatory phenotype. Conditions such as hyperglycaemia, steroid overuse and high levels of iron and ketone bodies upregulate the expression of GRP78, which, besides acting as a cofactor in viral entry, binds to spore-coating CotH3 invasin on the fungal surface and favours invasion of nasal epithelial cells by Mucorales. Similarly, the interaction of CotH7 with integrin α3β1 in alveolar epithelial cells contributes to the progression of pulmonary mucormycosis. The production of mucoricin by Mucorales hyphae promotes vascular damage that allows the enhanced recruitment of leucocytes from the bloodstream into the tissue, thereby favouring the hyperinflammatory state.
The severe lymphopenia and lymphocyte dysfunction typically observed in COVID-1986 may also influence the development of fungal co-infections. Severe lymphocytopenia was among the factors included in a prognostic model that predicted the development of invasive mould disease in haematological patients87. Moreover, Mucorales-specific T cells are detected in patients with mucormycosis88 and impairments in T cell responses during COVID-19 are aggravated in patients with diabetes89 at high risk of CAM. These observations suggest that the severely impaired cellular immunity in COVID-19 may increase susceptibility to secondary fungal infections.
In contrast to the general hyperinflammation phenotype, patients with COVID-19 display a remarkably decreased type I IFN (IFN-α and IFN-β) response90, resulting in part from the ability of SARS-CoV-2 to inhibit protein translation and abolish the type I IFN-dependent induction of IFN-stimulated genes91 (Fig. 4). Impaired IFN response was also associated with the presence of neutralizing autoantibodies against type I IFNs, which could negate their antiviral activity, in approximately 10% of patients with severe disease92. Importantly, type I IFNs can prime human dendritic cells in promoting type 1 T helper responses against Aspergillus93 and are key drivers of type III IFNs (IFN-λ), which are critical instructors of neutrophil-mediated antifungal responses94. In fact, high levels of IFN-λ, and to a lesser extent of type I IFN, were detected in the upper airways of patients with mild COVID-19 compared with those with severe disease95; this might help explain the increased susceptibility of the latter patients to fungal co-infections. In contrast, excessive or prolonged production of IFN-λ can hamper tissue repair during influenza or SARS-CoV-2 infection and ultimately increase susceptibility to secondary infections96,97.
Recognition of the influenza virus by TLR7 promotes type I IFN production by dendritic cells98; genetic variants in TLR7 that underlie the transcriptional downregulation of type I IFN signalling were found to predispose to COVID-19 in young males99. Strikingly, TLR7 expression was found to suppress the effector functions of macrophages against Aspergillus and aggravate pulmonary disease100, suggesting that, despite the essential role of type I IFNs in antifungal immunity, TLR7 signalling may be largely dispensable for protection from CAPA101.
While the sustained innate immune function leading to overt hyperinflammation is probably the main mechanism predisposing to CAPA, Mucorales species are instead endowed with specific virulence factors that may explain their preferential incidence among patients with COVID-19. Recent advances in genomics and transcriptomics coupled with the ability for gene manipulation technologies102,103,104,105,106 have enhanced our understanding of the pathogenesis of mucormycosis as it relates to angioinvasion and tissue necrosis and can provide an explanation for the development of CAM. Specifically, Mucorales universally express CotH invasins104,107 that allow inhaled spores to bind to the glucose-regulated protein 78 (GRP78) host receptor of nasal epithelial72 and endothelial cells108. It is also noted that both CotH proteins and GRP78 are highly upregulated during hyperglycaemia and ketoacidosis, which also increase the growth of the fungus via acquiring elevated available serum iron while reducing the host immune response109 (Fig. 4). The increased expression of the fungal CotH and host GRP78 in nasal epithelial cells leads to the inhaled spores being deposited in the sinus, causing ROM or ROCM rather than causing pulmonary infection via CotH/integrin α3β1 interactions72 (Fig. 4).
It is noteworthy that GRP78 synthesis is upregulated in patients with COVID-19 as a result of the virus-induced endoplasmic reticulum stress cascade110. Biochemical analyses have shown that GRP78 can form a complex with SARS-CoV-2 spike protein and ACE2 to act as a host auxiliary factor for SARS-CoV-2 entry and infection of host cells111. Importantly, treatment of lung epithelial cells with a humanized monoclonal antibody (hMAb159) that blocks the activity of endoplasmic reticulum chaperones was found to deplete GRP78 and reduce ACE2 expression on the cell surface, as well as viral entry and infection in vitro111. In a murine model of pulmonary mucormycosis, the interaction of CotH7 with the integrin β1 receptor on alveolar epithelial cells promoted the activation of the epidermal growth factor receptor (EGFR) and mediates host cell invasion72 (Fig. 4). This process may be of particular relevance to the pathogenesis of CAM since upregulation of the EGFR pathway in experimental SARS-CoV infection drives enhanced lung disease and fibrosis112. Infection of colonic epithelial cells by SARS-CoV-2 also demonstrated that the activation of EGFR promotes viral replication113. On the other hand, the toxin mucoricin inhibits protein synthesis and induces inflammation, vascular permeability and hypovolaemic shock and organ necrosis in mouse models of mucormycosis69. Therefore, vascular endothelial injury, further driven by COVID-19-associated inflammation, may also represent an important susceptibility feature of CAM.
The activation of a nutritional immunity programme in macrophages to restrict intracellular iron availability and inhibit fungal growth is an essential mechanism required to counter infection with Mucorales114. IL-6 production during COVID-19-associated hyperinflammation and in poorly controlled diabetes stimulates ferritin production, which results in increased levels of free iron in patients with COVID-1917. As Mucorales species require free iron for their biological processes, iron availability might therefore represent a critical mechanism involved in the pathogenesis of CAM115. This is illustrated in patients with COVID-19 and ketoacidosis or renal failure undergoing deferoxamine chelation116, where the circulating levels of free iron are further augmented. In addition, the impairment of neutrophil migration, ingestion and phagolysosome fusion caused by corticosteroids117, and the dysbiosis exerted by the intensive use of antibiotic treatment may also amplify the risk for CAM. SARS-CoV-2 can also infect human pancreatic β cells118 and promote morphological, transcriptional and functional alterations that result in reduced numbers of insulin-secretory granules and impaired glucose-stimulated insulin secretion119. Although it is currently not known whether the ability to infect β cells is a feature specific of the D614G variant of SARS-CoV-2 used in these studies, it is tempting to speculate that the escalating incidence of CAM cases during the second wave of the pandemic could be attributed, at least in part, to this viral pathogenic mechanism of induction of hyperglycaemia and ketoaciodosis120.
Circulating monocytes from patients with COVID-19 with severe respiratory failure show a decreased expression of human leucocyte antigen DR121, which is considered a marker of immune paralysis, a feature often associated with Candida sepsis. This is in line with the observation that monocytes from patients with severe COVID-19 exhibit a decreased expression of the co-stimulatory CD80 molecule and a blunted cytokine response after whole-blood stimulation with C. albicans, but not Aspergillus fumigatus122. Moreover, it is worth noting that recognition of Candida RNA by TLR7 and the downstream production of type I IFN—both often compromised in patients with severe COVID-19—has been reported to play an essential role in host defence against experimental candidiasis123,124. Although these results suggest a possibly disturbed immune response towards C. albicans, the relevance of these mechanisms to susceptibility to CAC is unclear. Moreover, the lymphopenia typical of COVID-19 is probably not the cause for enhanced susceptibility to CAC, as patients with HIV experiencing low lymphocyte counts are also not more prone to develop invasive candidiasis. Therefore, while the generally altered numbers and activation profiles of immune cells may contribute to tissue damage and the severity of COVID-19 favouring the progression of fungal disease, available evidence suggests that classical clinical risk factors, rather than overt immune dysfunction, are the major drivers of susceptibility to CAC.
Immunomodulation
Due to the need to counter the hyperinflammation that characterizes severe COVID-19, most of the available options to treat severe COVID-19 are immunomodulatory drugs, including corticosteroids and cytokine blockers such as tocilizumab. Despite their relevance to COVID-19 management, these drugs hamper the activation of innate and adaptive antimicrobial responses and therefore represent important predisposing factors to secondary fungal infections. Corticosteroids promote functional impairments of several immune cells, including neutrophils and monocytes/macrophages, and also T cells117. For this reason, the use of high-dose systemic corticosteroids is a relevant risk factor for developing aspergillosis, also in the context of COVID-19125, even though a contribution of additional underlying risk factors such as chronic respiratory diseases might confound these associations8. Moreover, treating patients with diabetes and COVID-19 with high doses of corticosteroids exacerbates hyperglycaemia, which results in augmented fungal invasion and impaired immune responses, thus generating the 'perfect storm' for increased CAM pathogenesis126.
In a large randomized controlled trial in critically ill patients with COVID-19, treatment with IL-6 receptor antagonists resulted in improved outcomes, including survival127. However, several reports have identified a link between the blockade of signals mediated by this critical cytokine in antifungal immunity and the risk of secondary fungal infections in critically ill patients with COVID-194,128,129. The reasons for this may be many in view of the pleiotropic effects of IL-6; however, in fungal sepsis, loss of IL-6 signals was recently found to impair the activation of LC3-associated phagocytosis, a molecular process that is essential for fungal killing by monocytes and macrophages130. For this reason, clinical trials are required to address the benefit of the combined use of IL-6 receptor antagonists and antifungal prophylaxis in patients with severe COVID-19.
Besides IL-6, another major inflammatory pathway in COVID-19 involves inflammasome activation and the consequent release of IL-1β131. Although inflammasome activation is generally required for protective responses to fungal pathogens132,133, inflammasome-dependent inflammation may become pathogenic if uncontrolled134,135. Type I IFNs can act as negative regulators of inflammasome activation and IL-1β production in response to fungal pathogens136, which suggests that the impaired type I responses in COVID-19 may facilitate aberrant inflammasome activation and the development of fungal co-infections. A recent phase III trial demonstrated that the early treatment of COVID-19 with the IL-1 receptor antagonist anakinra decreased mortality and shortened hospital stay137. Whether this therapeutic strategy could prevent the development of co-infections or instead potentiate susceptibility to them requires further investigation but IL-1 receptor blockade has been shown to ameliorate inflammation and restrain susceptibility to experimental aspergillosis in both chronic granulomatous disease135 and cystic fibrosis134.
Although the clinical benefit of immunomodulatory therapies in the treatment of COVID-19 is well established nowadays, recent evidence suggests collateral effects on susceptibility to fungal infections. Although in-depth clinical studies are required, these observations suggest that immunomodulation in critically ill patients with COVID-19 should be carefully tailored to the disease stage, for example, early immunosuppression in the ARDS stage and immune-enhancing strategies to rescue late-stage immunodepression, to minimize the risk of secondary infections.
Immunomodulatory treatment
Besides systemic antifungal treatment21,48,138, the management of COVID-19-associated IFIs should ideally also consider adjunctive host-directed therapies aimed at resolving immunopathology, while maintaining a balanced immune response required to adequately eliminate the invading fungus. Therefore, although several immune-dampening therapies have been explored in COVID-19 to reduce systemic inflammation, administration of immune boosting agents should also be considered. The most important clinical trials of immunotherapy in COVID-19 are discussed in detail in van de Veerdonk et al.139.
In the later disease stages where immune paralysis is often present, administration of recombinant IFN-γ or type I IFNs could prove beneficial140. IFN-γ has been shown to restore immune function, including antigen presentation and the capacity of immune cells to produce proinflammatory cytokines, in a case series of patients with fungal sepsis141. More recently, two case series reported a potentially beneficial effect of IFN-γ in patients with severe COVID-19, using positive-to-negative viral culture conversion and monocyte human leukocyte antigen-DR isotype (HLA-DR) expression and total lymphocyte counts as primary end points142,143. Given the well-established positive effects of IFN-γ on antifungal immunity, these observations support the possibility that, besides a direct effect on COVID-19, IFN-γ therapy may also have clinical value in preventing or treating fungal co-infections. Of note, large prospective trials in the context of influenza-associated aspergillosis are ongoing not only to assess the therapeutic potential of IFN-γ but also to identify biomarkers that could allow the identification of patients who would benefit the most from this immunotherapy. Such efforts are thus expected to pave the way towards a new generation of adjunct host-directed medicine approaches to manage fungal infections more efficiently.
Other preclinical investigational therapies include fungus-directed immunotherapies aiming to boost the host immune response to infections. These include antibodies targeting components of the cell wall or toxins. For example, β-1,3-glucans are major components of many pathogenic fungi including A. fumigatus and Candida. A murine monoclonal antibody specific for β-1,3-glucans, 2G8 (an IgG2b), was shown to be protective in rodent models of C. albicans infections144. Recently, a humanized version of 2G8 monoclonal antibody (H5K1) was shown to inhibit the growth of C. auris and acted synergistically with either caspofungin or amphotericin B in time-kill curves and enhanced opsonophagocytosis by macrophages in vitro145. Although it is currently unknown whether H5K1 protects against experimental fungal infections, this humanized monoclonal antibody has the potential to protect against several infections since it binds to C. albicans, C. auris, Candida glabrata, A. fumigatus and Fusarium solani145. Also, a fully human antibody-targeting C. albicans cell surface Hyr1p was shown to bind to C. auris and enhance its phagocytosis by J774.1 macrophage cells. This antibody protects mice from C. albicans disseminated infection146.
Another passive immunization approach was shown to enhance treatment outcome against mucormycosis. A murine monoclonal antibody (C2, an IgG1) targeting the cell-surface invasin CotH3 was shown to be protective in mouse models of severe mucormycosis especially when combined with antifungal drugs147. A humanized version of C2, VX01, had a tenfold increase in binding affinity to recombinant CotH3p and was equally protective against murine mucormycosis when compared with the mouse C2 monoclonal antibody and had safe profiles in human tissue cross-reactivity studies148. VX01 is currently in manufacturing. Similarly, polyclonal antibodies targeting mucoricin toxin enhanced median survival time and prolonged overall survival of mice with mucormycosis through reduction of tissue damage69.
Finally, monoclonal antibodies against Als3p from C. albicans blocked the interaction of the fungus with host cells149; vaccination with rAls3-N was shown to protect from lethal disseminated candidiasis150. Likewise, bioinformatic and immune-profiling studies identified three orthologues on the C. auris cell wall with three-dimensional structural and functional similarity with C. albicans Als3p. Anti-Als3p antibodies bound to C. auris prevented biofilm formation and enhanced the opsonophagocytic activity of macrophages. Importantly, the experimental vaccine NDV-3A (recombinant Als3p-N formulated with alum) significantly improved overall survival of mice infected with haematogenously disseminated C. auris and acted synergistically with micafungin151.
Conclusion and future perspectives
Despite the broad importance and socio-economic impact of medical mycology, research on fungal infections has lagged behind compared to other pathogens. The recent SARS-CoV-2 pandemic has highlighted the importance of fungal infections for morbidity and mortality, even in previously immunocompetent populations who suffer from viral disease. IPA in patients in the ICU had been frequently overlooked before, as shown in a pre-COVID-19 autopsy study, where 2.8% of autopsies in patients in the ICU revealed IPA; only 40% of those autopsy cases had been diagnosed pre-mortem152.
To estimate incidence and set a standard for epidemiological studies and future clinical trials, it will be important to define consensus criteria that are used homogeneously for the classification of IFIs. As a breakthrough, global efforts by mycologists have resulted in the development of broadly accepted consensus criteria for CAPA in late 202021. To reduce morbidity and mortality from opportunistic IFIs, a general awareness of the relevance of fungal co-infection is critical to avoid delays in diagnosis and treatment. In the absence of antifungal prophylaxis, screening of COVID-19 patients for fungal diseases is essential, which optimally—at least for CAPA and CAM—involves repeated testing of samples from the lower respiratory tract in case of clinical suspicion22, while blood cultures remain the criterion standard for CAC. The difficulties to diagnose pulmonary or gastrointestinal CAM may contribute to the overwhelming proportion of ROM/ROCM in CAM cases reported from India and other low- and middle-income countries (Box 1), while in high-income countries, pulmonary CAM is reported in about 50% of cases14. Culture and histology remain central for diagnosing CAM and CAC48,53, but new diagnostics including Mucorales PCR from blood153,154

Comments
Post a Comment