Cutaneous Manifestations of HIV: Overview, Manifestations by HIV Disease Stage, Manifestations in HIV-Infected ...
Ancient Malaria Genome From Roman Skeleton Hints At Disease's History
March 15, 2024
3 min read
Ancient Malaria Genome from Roman Skeleton Hints at Disease's History
Genetic information from ancient Roman remains is helping to reveal how malaria has moved and evolved alongside people
Malaria, an endemic disease caused by hematozoic parasites (Plasmodium falciparum) transmitted by the blood to humans through the bite of the female anophele mosquito.
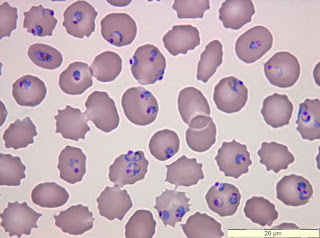
Credit:BSIP SA/Alamy Stock Photo
Genetics
Researchers have sequenced the mitochondrial genome of the deadliest form of malaria from an ancient Roman skeleton. They say the results could help to untangle the history of the disease in Europe.
It's difficult to find signs of malaria in ancient human remains, and DNA from the malaria-causing parasite Plasmodium rarely shows up in them. As a result, there had never been a complete genomic sequence of the deadliest species, Plasmodium falciparum, from before the twentieth century — until now. "P. Falciparum was eliminated in Europe a half century ago, and genetic data from European parasites — ancient or recent — has been an elusive piece in the puzzle of understanding how humans have moved parasites around the globe," says Daniel Neafsey, who studies the genomics of malaria parasites and mosquito vectors at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts.
Malaria has long been a leading cause of human deaths. "With the development of treatments such as quinine in the last hundreds of years, it seems clear [humans and malaria] are co-evolving," says Carles Lalueza Fox, a palaeogenomicist at the Institute of Evolutionary Biology in Barcelona, Spain. "Discovering the genomes of the ancient, pre-quinine plasmodia will likely reveal information about how they have adapted to the different anti-malarial drugs."
On supporting science journalismIf you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Ancient pathogenThere are five malaria-causing species of Plasmodium, which are thought to have arisen in Africa between 50,000 and 60,000 years ago, and then spread worldwide. Most researchers agree that they reached Europe at least 2,000 years ago, by the time of the Roman Empire.
Plasmodium falciparum "has significantly impacted human history and evolution", says Neafsey. "So, that makes it particularly important to discover how long different societies have had to deal with [it], and how human migration and trade activities spread it."
Researchers can glean valuable information about the origin, evolution and virulence of the parasite from DNA extracted from the ancient remains of infected people. But it is difficult to know where to look: it is not always obvious whether a person was infected with Plasmodium, and whether DNA can be recovered depends on how well it has been preserved.
In a preprint posted on the server bioRxiv, a team of researchers led by a group at the University of Vienna identified the first complete mitochondrial genome sequence of P. Falciparum from the bones of a Roman who lived in Italy in the second century AD, known as Velia-186.
Plasmodium falciparum had been detected in Velia-186 in a previous study. The authors of the latest preprint extracted the parasite's DNA from the body's teeth, and were able to identify 5,458 pieces of unique genetic information that they combined to get a sequence covering 99.1% of the mitochondrial genome. They also used software to compare the genome with modern samples, and found that the Velia-186 sequence is closely related to a group of present-day strains found in India.
Carried by migrationThe researchers say their findings support a hypothesis that P. Falciparum spread to Europe from Asia around at least 2,000 years ago. The Indian strains "were already present in Europe [then]; thus, a potential arrival with globalization episodes such as the Hellenistic period — when it is first described by Greeks — seems plausible", says Lalueza Fox.
Neafsey says the work is a "technical tour de force" and an interesting addition to the limited field of ancient malaria genomics. But he adds that the results should be interpreted with caution because there are only a few samples, and points out that a genome sequence from DNA in the parasite's cell nuclei, rather than its mitochondria, "might indicate a more complex story of parasite movement among ancient human populations".
Lalueza Fox suggests exploring other potential sources of Plasmodium DNA, such as old bones, antique medical equipment and even mosquito specimens in museums. "The integration of genetic data from these heterogeneous sources will provide a nuanced view of this disease," he says. "It would be interesting to see what lessons we can learn from the past on the strains and dispersals of this pathogen."
This article is reproduced with permission and was first published on March 13, 2024.
Insights Into A Malarial Parasite — Plasmodium Falciparum's Genetic Arsenal
New 'copy-paste' mechanism in genetics have been identified by researchers at EMBL's European Bioinformatics Institute (EMBL-EBI) in a malaria parasite Plasmodium falciparum as per a study published in the journal PLOS Biology (1✔ ✔Trusted SourceRole for gene conversion in the evolution of cell-surface antigens of the malaria parasite Plasmodium falciparumGo to source). Malaria is most commonly transmitted through the bites of female Anopheles mosquitoes infected with P. Falciparum.The latest world malaria report states that in 2022, there were an estimated 249 million malaria cases and over 600,000 malaria deaths across the globe.
94% of malaria cases and 95% of malaria deaths are found in Africa, with infants, pregnant women, travellers, and people with HIV/AIDS being at higher risk.
'Plasmodium falciparum, the deadliest malaria parasite, utilizes gene conversion to rapidly diversify surface protein genes, aiding its evasion of the human immune system and explaining the presence of genetic diversity hotspots. #malaria #parasite #geneticdiversity 'Advertisement
Gene Conversion and Immune Resistance The new study provides key insights into the evolutionary history of P. Falciparum through the analysis of two genes that encode surface proteins critical to immune evasion. The genes in question are DBLMSP and DBLMSP2.These findings deepen our understanding of how the malaria parasite has evolved and could help to inform new approaches to vaccine development, offering hope for more effective prevention methods against a disease that continues to impact millions globally.
Usually, the sequence of an individual's gene is inherited from their parents, but in some circumstances, part of a gene sequence can be copied between different genes on the same DNA molecule – this is known as non-allelic gene conversion.
This process has been linked to the evolution of important gene families, including those involved in the functioning of the human immune system.
Did You Know? Approximately 94% of malaria cases and deaths occur in sub-Saharan Africa, primarily impacting young children and pregnant women. One of this study's key discoveries is that gene conversion takes place between the P. Falciparum DBLMSP and DBLMSP2 genes and results in increased genetic diversity within the surface proteins of the parasite.
Since these proteins are exposed to, and interact with our immune system, they are potential vaccine targets, and a fuller understanding of their genetic diversity could be very valuable for vaccine design.
"The discovery of 'copy-paste' genetics within malaria's DNA reveals the impact of an underestimated evolutionary mechanism," said Brice Letcher, Postdoctoral Researcher at the Laboratory for Biology and Modelling of the Cell (LBMC, France) and former PhD student at EMBL-EBI.
"Here we show that gene conversion was a potentially important strategy behind malaria's ability to adapt and thrive in humans, including possibly to evade the human immune system. Understanding this genetic flexibility offers new perspectives on malaria's persistence in and adaptation to the human host."
Advertisement
Lethality Code of Malaria Parasites Any immune-interacting protein is potentially a vaccine target, but knowledge of global genetic diversity is an important requirement for vaccine development.For example, influenza and SARS-CoV-2 vaccines are developed based on the knowledge of how their genomes have evolved.
However, the very unusual hotspots of genetic diversity in the P. Falciparum DBLMSP and DBLMSP2 genes are so extreme that current algorithms for mapping genetic variants failed to capture them, leaving researchers unaware of a large proportion of the variation in these genes.
To address this, the researchers developed new bioinformatics software that uses genome graphs and analysed a broad sample of parasites from 29 countries.
This new approach revealed a wide range of previously hidden variants, and with these, they were able to demonstrate that multiple gene conversion events had occurred. These new variants, available for download from the website linked to the study, provide a valuable resource for the malaria research community.
"Genome graphs are a great bioinformatics method to help us decode the complex genetic landscapes arising from the interplay between pathogens and human hosts," said Sorina Maciuca, co-author and former PhD student in the Iqbal group and Genomics Data Scientist at Genomics England.
"They allow us to take into account a broader spectrum of genetic diversity and obtain new insights into how pathogens like P. Falciparum evolve and evade our immune defences."
Advertisement
What are genome graphs? The traditional approach in genomics is to define one reference genome and describe any other genome as a set of small differences from this reference. This does not work well when genomes differ too much.Genome graphs take a population of genomes and build an ensemble reference which is aware of all of the genetic variation in the species.
"This research provides a comprehensive map of genetic diversity of these two fascinating genes in P. Falciparum," said Zamin Iqbal, Group Leader at EMBL-EBI and Professor of Algorithmic and Microbial Genomics at the University of Bath.
"We have been trying to understand the unusual patterns in these genes for almost a decade now, and our best hypothesis had been that the really different "versions'' of the gene were being preserved by natural selection, for unknown reasons. We have shown here that, in fact, this copying mechanism – gene conversion – has been repeatedly creating these anomalous different "versions" of the genes. This data not only enhances our grasp of malaria's biology, but also will be valuable to researchers across the world studying these genes and their interaction with our immune system."
Reference:
FDA Approves Roche's Cobas Malaria Test, Designed To Screen For Malaria In Potential Blood Donors
Action marks the first FDA-approved blood screening test for malaria.
Image Credit: Adobe Stock Images/Dr_Microbe
Roche announced that the FDA has officially approved the Cobas Malaria test, focused on inspecting blood donors for malaria, aiming to enhance the safety of blood supply. The test, which screens blood samples for five species of Plasmodium parasites, is the first of its kind approved for this purpose and aims to reduce the risk of malaria transmission through transfusions. Roche stated that it expects the test to be available in the United States at some point during the second quarter of this year.1
"As the first FDA-approved blood screening test for malaria, this represents an important step forward in safeguarding the global supply of donated blood," said Matt Sause, CEO, Roche Diagnostics, in a press release. "The approval of cobas Malaria represents a significant advancement in malaria detection, offering healthcare professionals a reliable tool for donor screening and improving the safety of patients worldwide."
According to the company, close to half of the global population was at risk for malaria in 2022, with substantial rates of mortality, especially in Africa. Other areas that reported significant numbers included Southeast Asia, the Eastern Mediterranean, the Western Pacific, and the Americas. Prior tests on the market weren't able to pick up on malaria transfusion risks.1
According to the World Health Organization (WHO), children under 5 years of age made up 80% of all malaria-related deaths in Africa. Symptoms usually manifest around 10-15 days after getting bitten by an infected mosquito. Common symptoms of the disease include:
"Malaria mostly spreads to people through the bites of some infected female Anopheles mosquitoes," states WHO. "Blood transfusion and contaminated needles may also transmit malaria. The first symptoms may be mild, similar to many febrile illnesses, and difficulty to recognize as malaria. Left untreated, P. Falciparum malaria can progress to severe illness and death within 24 hours."
There are also multiple ways to prevent malaria, such as avoiding mosquitos and consulting a physician on medication. WHO also suggested multiple ways of avoiding mosquito bites, including netting mosquitos when sleeping in areas in which they are present, wearing protective clothing, and using repellant.2
"Since October 2021, WHO has recommended broad use of the RTS,S/AS01 malaria vaccine among children living in regions with moderate to high P. Falciparum malaria transmission. The vaccine has been shown to significantly reduce malaria, and deadly severe malaria, among young children," WHOexplained. "In October 2023, WHO recommended a second safe and effective malaria vaccine, R21/Matrix-M. The availability of two malaria vaccines is expected to make broad-scale deployment across Africa possible."
References
1. Roche receives FDA approval for the first molecular test to screen for malaria in blood donors. Roche. March 26, 2024. Accessed March 27, 2024. Https://www.Roche.Com/media/releases/med-cor-2024-03-26
2. Malaria. WHO. December 4, 2023. Accessed March 27, 2024. Https://www.Who.Int/news-room/fact-sheets/detail/malaria#:~:text=Disease%20burden,to%20610%20000%20in%202021.

Comments
Post a Comment