What Complications Can Occur With HIV?
11 Diseases That Can Pass From Animals To Humans
Anthrax can be transmitted by animals like cattle, sheep, and deer.Though anthrax is most commonly associated with bioterrorism, this rare disease is actually caused by the bacteria Bacillus anthracis and can be spread by both domestic and wild animals. People can become infected by anthrax if they breathe in anthrax spores, consume contaminated food or water, or are exposed through broken skin.
Symptoms can vary based on the way someone was exposed but often include blisters or sores with a black center, fever, headache, nausea, body pain, shortness of breath, confusion, and painful swallowing. Treatment normally involves taking antibiotics, antitoxin, or both. Anthrax is a serious condition that can be fatal.
According to the World Health Organization, cattle, sheep, goats, antelope, and deer are the most common source of animal-transmitted anthrax. People who work with animals or animal products are most at risk of exposure, though contracting anthrax is still extremely rare.
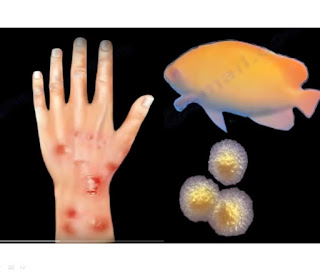
You can contract the orf virus from goat yoga and petting zoos.The orf virus generally affects small ruminants like sheep and goats, but it can be passed to humans through contact with infected animals or contaminated items.
"Orf typically presents with a pustule that ultimately progresses into a sore, usually on the hand. There was an increase in orf diagnoses as goat yoga became popular, but it can occur after visiting a farm or petting zoo as well," board-certified dermatologist Dr. Susan Bard told INSIDER.
You can catch orf from petting infected animals, though the virus can also spread through bites or equipment. The resulting sores can be painful but usually heal on their own in about six weeks as long as they are kept clean, dry, and free of bacterial infection. Orf can't be passed between humans.
Though orf normally isn't dangerous, some more serious zoonotic diseases can sometimes look like orf, including anthrax. It's important to get checked out by a doctor if you suspect you've contracted an infection from a sheep or goat.
Giardia is a parasite you can get from your pets.Giardia is an intestinal parasite that lives in the infected feces of animals or humans. It can be spread through contact with anything that has been exposed to the infected waste, including water, ice, soil, and household items.
"A pet can track giardia through your home after it's infected. If your pet has symptoms of diarrhea, a rumbly tummy or vomiting you need to see your veterinarian to confirm a diagnosis," veterinarian Dr. Jim D. Carlson told INSIDER.
In humans, giardia can cause diarrhea, gas, greasy stools that float, nausea, and stomach pain. Children and pregnant women infected with giardia are especially prone to severe dehydration. Symptoms generally last between two and six weeks and can start up to three weeks after infection. The infection is generally treated with prescription drugs.
Dr. Carlson advised that floors, furniture, toys, and bedding should all be thoroughly cleaned after contact with an infected pet. It's also important to retest your pet following treatment for giardia to make sure the infection has been fully cleared.
Toxoplasmosis is spread by cats and can linger in your system for years.Toxoplasmosis is sometimes confused with Bartonella, but though these conditions are both spread by cats, they're caused by different organisms. Toxoplasmosis is caused by the parasite Toxoplasma gondii and can be caught by contact with infected cat feces or urine. The parasite can't pass through intact skin, so infection usually occurs through the mouth or an open wound.
"The disease is generally quite mild, but can be serious in individuals who are pregnant, going through chemotherapy, or have a compromised immune system. Symptoms can include fatigue, muscle aches, headaches, and fever that can last over a month," infectious disease specialist Dr. Nidhi Ghildayal told INSIDER.
In otherwise healthy people, most cases clear up without treatment within a few months. However, severe cases of toxoplasmosis can cause damage to the brain, eyes, or other organs. The disease can also reactivate years after the initial infection.
To guard against toxoplasmosis, Dr. Carlson told INSIDER that cat owners should keep litter boxes clean and always use gloves and cleaning agents when handling cat feces. Soil can also become infected, so wear gloves if you're gardening in an area frequented by cats.
It's possible to catch a bacterial infection from pet fish. Parrot fever can be contracted from pet birds.Psittacosis, also known as parrot fever, is an infection caused by the bacteria Chlamydia psittaci.
"Psittacosis can be spread when individuals inhale respiratory fluids or feces from parrots, pigeons, macaws, parakeets, and other birds that carry the bacterial infection. Birds may not show symptoms, so the disease can be hard to detect," said Dr. Ghildayal
Psittacosis can cause fever, headache, and a dry cough in humans. Though the infection is rarely serious and can usually be easily treated with antibiotics, it can lead to pneumonia in some people.
Keep bird cages as clean as possible, avoid bird overcrowding, and make sure feces and other waste can't pass between cages to help prevent Psittacosis. You should also wear gloves and masks when handling infected birds and cages.
Ringworm is a fungus that can spread from dogs and cats.Dr. Bard told INSIDER that one of the most common zoonotic skin infections is ringworm. Despite its name, ringworm is actually a fungal infection and is usually contracted from cats, dogs, and other pets. It causes a distinctive circular rash that is usually itchy and red.
"Zoonotic strains of ringworm tend to be more inflammatory than other strains. It is easily treated with topical or oral antifungals," said Dr. Bard.
Ringworm can live on household items like bedding, furniture, and clothing. Though you can get it from animals, the fungus can also be spread from person to person. Wear gloves when touching items that have come into contact with a ringworm-infected person or pet and always wash your hands after handling animals.
You can catch Salmonella from reptiles and amphibians.Salmonella is most commonly associated with contaminated food, but Dr. Fiorito told INSIDER that you can actually contract a Salmonella infection through contact with reptiles like lizards, snakes, and turtles or amphibians such as frogs or salamanders.
"A Salmonella outbreak tied to bearded dragons spread to 31 states from 2012 to 2014. It was a rare strain that was antibiotic-resistant. The majority of those sickened were five years and younger, but fortunately, there were no deaths," said Dr. Fiorito.
Symptoms of Salmonella in healthy adults are usually limited to diarrhea, abdominal cramps, fever, and vomiting and normally resolve without treatment. However, people with compromised immune systems such as children and the elderly can become seriously or fatally ill. Talk to your healthcare provider if you suspect you have a Salmonella infection.
Leptospirosis can be passed from farm animals to people.Leptospirosis, or Weil's disease, is a bacterial infection that typically affects farmers and those who work directly with livestock like pigs, sheep, and cattle.
"Leptospirosis is typically transmitted through direct contact with infected animal urine. Symptoms are normally mild and flu-like. More severe infections can lead to yellowing of eyes or the skin, or liver and kidney failure," Dr. Adeline Peters, general practitioner and lead physician at DoctorOnCall, told INSIDER.
Horses, dogs, and rodents can also carry the infection, which is often passed to humans through contaminated water, contact with broken skin, or the mucous membranes in the eyes, nose, or mouth. With antibiotic treatment, people normally heal in three weeks to a month. Without treatment, recovery may take months.
Rabies is spread through animal saliva and is a medical emergency.One of the most notorious zoonotic diseases is rabies, a virus that is almost always lethal.
"This disease infects the central nervous symptom and results in death if not medically treated with urgency. Rabies is often spread through the saliva of an infected raccoon, coyote, bat, skunk, fox, dog, or cat — in fact, any mammal can spread the disease," explained Dr. Ghildayal.
Early symptoms of rabies include headache, weakness, and a prickling or itching at the infection site, according to the WHO. As the infection progresses, fever, confusion, agitation, and seizures may develop. People with advanced rabies may also exhibit hydrophobia, or fear of water.
Unfortunately, rabies is almost always fatal once symptoms develop. The CDC reported that there have only ever been 10 documented cases of recovery from rabies, and only two of those people survived without immediate treatment.
"Travelers need to be wary in foreign countries about petting local animals, such as stray dogs. A person can get rabies without being bitten, such as when the saliva of the animal infiltrates cuts on the neck or face," warned Dr. Fiorito.
It's extremely important that anyone who may have possibly been exposed to rabies gets treated as soon as possible. Treatment usually consists of four vaccines administered over 30 days. According to the NHS, post-exposure treatment is nearly 100% effective if received immediately after exposure.
Death And Doctor Hornbook
Some books are lies frae end to end, And some great lies were never penn'd: Ev'n ministers they hae been kenn'd, In holy rapture, A rousing whid at times to vend, And nail't wi' Scripture. But this that I am gaun to tell, Which lately on a night befell, Is just as true's the Deil's in hell Or Dublin city: That e'er he nearer comes oursel' 'S a muckle pity. The clachan yill had made me canty, I was na fou, but just had plenty; I stacher'd whiles, but yet too tent aye To free the ditches; An' hillocks, stanes, an' bushes, kenn'd eye Frae ghaists an' witches. The rising moon began to glowre The distant Cumnock hills out-owre: To count her horns, wi' a my pow'r, I set mysel'; But whether she had three or four, I cou'd na tell. I was come round about the hill, An' todlin down on Willie's mill, Setting my staff wi' a' my skill, To keep me sicker; Tho' leeward whiles, against my will, I took a bicker. I there wi' Something did forgather, That pat me in an eerie swither; An' awfu' scythe, out-owre ae shouther, Clear-dangling, hang; A three-tae'd leister on the ither Lay, large an' lang. Its stature seem'd lang Scotch ells twa, The queerest shape that e'er I saw, For fient a wame it had ava; And then its shanks, They were as thin, as sharp an' sma' As cheeks o' branks. "Guid-een," quo' I; "Friend! Hae ye been mawin, When ither folk are busy sawin!" I seem'd to make a kind o' stan' But naething spak; At length, says I, "Friend! Whare ye gaun? Will ye go back?" It spak right howe, - "My name is Death, But be na fley'd." - Quoth I, "Guid faith, Ye're maybe come to stap my breath; But tent me, billie; I red ye weel, tak care o' skaith See, there's a gully!" "Gudeman," quo' he, "put up your whittle, I'm no designed to try its mettle; But if I did, I wad be kittle To be mislear'd; I wad na mind it, no that spittle Out-owre my beard." "Weel, weel!" says I, "a bargain be't; Come, gie's your hand, an' sae we're gree't; We'll ease our shanks an tak a seat- Come, gie's your news; This while ye hae been mony a gate, At mony a house." "Ay, ay!" quo' he, an' shook his head, "It's e'en a lang, lang time indeed Sin' I began to nick the thread, An' choke the breath: Folk maun do something for their bread, An' sae maun Death. "Sax thousand years are near-hand fled Sin' I was to the butching bred, An' mony a scheme in vain's been laid, To stap or scar me; Till ane Hornbook's ta'en up the trade, And faith! He'll waur me. "Ye ken Hornbook i' the clachan, Deil mak his king's-hood in spleuchan! He's grown sae weel acquaint wi' Buchan And ither chaps, The weans haud out their fingers laughin, An' pouk my hips. "See, here's a scythe, an' there's dart, They hae pierc'd mony a gallant heart; But Doctor Hornbook, wi' his art An' cursed skill, Has made them baith no worth a fart, Damn'd haet they'll kill! "'Twas but yestreen, nae farther gane, I threw a noble throw at ane; Wi' less, I'm sure, I've hundreds slain; But deil-ma-care, It just play'd dirl on the bane, But did nae mair. "Hornbook was by, wi' ready art, An' had sae fortify'd the part, That when I looked to my dart, It was sae blunt, Fient haet o't wad hae pierc'd the heart Of a kail-runt. "I drew my scythe in sic a fury, I near-hand cowpit wi' my hurry, But yet the bauld Apothecary Withstood the shock; I might as weel hae tried a quarry O' hard whin rock. "Ev'n them he canna get attended, Altho' their face he ne'er had kend it, Just shite in a kail-blade, an' sent it, As soon's he smells 't, Baith their disease, and what will mend it, At once he tells 't. "And then, a' doctor's saws an' whittles, Of a' dimensions, shapes, an' mettles, A' kind o' boxes, mugs, an' bottles, He's sure to hae; Their Latin names as fast he rattles as A B C. "Calces o' fossils, earths, and trees; True sal-marinum o' the seas; The farina of beans an' pease, He has't in plenty; Aqua-fontis, what you please, He can content ye. "Forbye some new, uncommon weapons, Urinus spiritus of capons; Or mite-horn shavings, filings, scrapings, Distill'd per se; Sal-alkali o' midge-tail clippings, And mony mae." "Waes me for Johnie Ged's Hole now," Quoth I, "if that thae news be true! His braw calf-ward whare gowans grew, Sae white and bonie, Nae doubt they'll rive it wi' the plew; They'll ruin Johnie!" The creature grain'd an eldritch laugh, And says "Ye needna yoke the pleugh, Kirkyards will soon be till'd eneugh, Tak ye nae fear: They'll be trench'd wi' mony a sheugh, In twa-three year. "Whare I kill'd ane, a fair strae-death, By loss o' blood or want of breath This night I'm free to tak my aith, That Hornbook's skill Has clad a score i' their last claith, By drap an' pill. "An honest wabster to his trade, Whase wife's twa nieves were scarce weel-bred Gat tippence-worth to mend her head, When it was sair; The wife slade cannie to her bed, But ne'er spak mair. "A country laird had ta'en the batts, Or some curmurring in his guts, His only son for Hornbook sets, An' pays him well: The lad, for twa guid gimmer-pets, Was laird himsel'. "A bonie lass-ye kend her name- Some ill-brewn drink had hov'd her wame; She trusts hersel', to hide the shame, In Hornbook's care; Horn sent her aff to her lang hame, To hide it there. "That's just a swatch o' Hornbook's way; Thus goes he on from day to day, Thus does he poison, kill, an' slay, An's weel paid for't; Yet stops me o' my lawfu' prey, Wi' his damn'd dirt: "But, hark! I'll tell you of a plot, Tho' dinna ye be speakin o't; I'll nail the self-conceited sot, As dead's a herrin; Neist time we meet, I'll wad a groat, He gets his fairin!" But just as he began to tell, The auld kirk-hammer strak the bell Some wee short hour ayont the twal', Which rais'd us baith: I took the way that pleas'd mysel', And sae did Death.MftG Is Crucial For Alcohol Metabolism Of Mycobacteria By Linking Mycofactocin Oxidation To Respiration
Mycobacteria are a metabolically versatile group of microorganisms that are able to utilize various organic compounds for their carbon and energy metabolism. Especially environmental mycobacteria like M. Smegmatis utilize for instance sugars, polyols, small organic acids, fatty acids, or sterols for their growth (1). While obligate pathogens like M. Tuberculosis, the causative agent of tuberculosis, are more limited in their menu, they still retain the ability to metabolize various nutrients available in the host organism (2, 3). Intriguingly, alcohols like ethanol are readily consumed by many mycobacterial species, but the metabolic pathway for ethanol consumption requires genes related to the biosynthesis of the unusual redox cofactor mycofactocin (MFT) (4, 5). Indeed, the inactivation of the mycofactocin biosynthetic gene cluster mftA-F (Figure 1A) showed a severe impact on the growth of M. Smegmatis and M. Marinum cultivated on ethanol as the sole carbon source. For M. Tuberculosis, a similar growth deficit on media containing ethanol and cholesterol was observed (5). In M. Smegmatis, it was shown that the gene MSMEG_6242, encoding a MFT-associated alcohol dehydrogenase (termed Mno or Mdo), is strictly required for alcohol utilization. Mno/Mdo was also demonstrated to catalyze methanol metabolism in the same organism (6) and involvement of mycofactocin in this process was suggested (7). Furthermore, a metabolomics study conducted in our lab revealed that MFT production in M. Smegmatis was significantly increased on ethanol as carbon source in comparison to a control cultivated on glucose (8). Recently, a homolog of Mdo was shown to be essential for MFT-dependent ethylene glycol oxidation in Rhodococcus jostii (9). All of this evidence supported the hypothesis that mycofactocin enables alcohol metabolism by acting as an electron acceptor of alcohol dehydrogenases (Figure 1B) (4, 5).
Figure 1:The mycofactocin redox system.(A) Schematic representation of the mft gene cluster of M. Smegmatis. MftA-F: MFT biosynthetic genes. MftR: TetR-like regulator. MftG: GMC oxidoreductase (subject of this study). (B) Chemical structures of MMFT-n (oxidized methylmycofactocin) and MMFT-nH2 (reduced form) and hypothetical scheme of MFT reduction by the ethanol dehydrogenase Mdo/Mno. The proposed mycofactocin dehydrogenase is the subject of this study. X: Unknown electron acceptor.
Although the role of MFT in alcohol metabolism is well established, further biological roles of mycofactocin appear to exist. Intriguingly, the MFT gene locus seems to be derepressed by long-chain fatty acid-CoA esters in M. Smegmatis, which bind to the repressor mftR (10). Furthermore, a comparison of the proteome of active and dormant cells revealed that mftD (Rv0694) was upregulated in the dormant state, which is associated with the persistence of M. Tuberculosis in macrophages (11). Lastly, a study on the impact of mycofactocin inactivation (ΔmftD) on M. Tuberculosis showed increased growth on glucose containing media as well as a decreased number of mycobacterial cells in a mouse model after the onset of hypoxia (12).
Mycofactocin is a ribosomally synthesized and post-translationally modified peptide (RiPP) (13). After translation, the precursor peptide MftA is bound by its chaperone MftB and undergoes several modifications until it matures into a redox-active molecule. More precisely, its C-terminal core peptide (Val-Tyr) is decarboxylated and cyclized by the radical SAM maturase MftC (14). The cyclized core peptide (AHDP) is liberated from the precursor by the peptidase MftE (15). The flavoenzyme MftD further deaminates AHDP to premycofactocin (PMFT) thus creating a redox-active ketoamide moiety (16). In vivo, mycofactocins exist in oligoglycosylated forms (MFT-n), where n denotes the number of β-1,4-linked glucose moieties. The glycosylation requires the presence of the glycosyltransferase MftF (8). In some mycobacteria, the glycosyl chain of MFT-n is further modified by methylation at the second sugar moiety (yielding MMFT-n) by the mycofactocin-associated methyltransferase MftM (17).
The reduction of M/MFT-n to M/MFT-nH2 during growth on ethanol is likely to be catalyzed by the above-mentioned alcohol dehydrogenase Mno/Mdo (5, 7). However, two important questions remained open concerning the mycofactocin system. First, it is unclear how MFT-nH2 is re-oxidized to MFT-n after reduction by Mno/Mdo or other enzymes (Figure 1B). Such a step would be crucial to regenerate the cofactor and allow for further electron transfer to terminal electron acceptors such as oxygen. Second, the role of the mftG gene (Figure 1A), the terminal gene in the mft gene cluster of mycobacteria, encoding a flavoenzyme of the glucose-methanol-choline (GMC) oxidoreductase superfamily remained unknown. GMC oxidoreductases are present in bacteria, fungi, plants, and insects (18, 19) and are known to catalyze redox reactions using a variety of different types of substrates and electron acceptors. Prominent examples are the fungal glucose oxidases, which are used in portable glucose biosensors for diabetes patients (20). Other important representatives are the alcohol oxidases from the methylotrophic yeast Pichia pastoris, which uses oxygen as an electron acceptor (21), or the choline dehydrogenase of E. Coli, which feeds electrons into the electron transport chain (22). We therefore hypothesized that MftG may be an oxidoreductase involved in mycofactocin-related metabolism (4). Here, we show that mftG (MSMEG_1428), encoding a flavoenzyme of the GMC family, is crucial for ethanol metabolism of the model organism Mycolicibacterium smegmatis. We further present evidence that MftG acts as a mycofactocin dehydrogenase and promotes MFT regeneration via electron transfer to the respiratory chain of mycobacteria.
Phylogeny and co-occurrence of mftG and mycofactocinPrimary sequence alignments with known members of the GMC family and AlphaFold (23, 24) prediction of the tertiary structure of MftG (WP_014877070.1) from M. Smegmatis MC2 155 (Figure 2A) confirmed that MftG is a protein of the GMC superfamily with an intact FAD binding pocket (Rossmann fold) and a conserved active site histidine (25). While these properties are general characteristics of the GMC superfamily, a hallmark of the MftG subfamily of GMC oxidoreductases is their tight genetic linkage to the mycofactocin biosynthetic gene cluster mftA-F (4). These MFT-associated GMC homologs are defined by three protein families. The first, TIGR03970.1 (dehydrogenase, Rv0697 family) describes proteins from various actinobacteria and includes the M. Smegmatis homolog. The other two families are TIGR04542 (GMC_mycofac_2) and NF038210.1 (GMC_mycofac_3). TIGR04542 is specific to the Gordonia genus, while NF038210.1 is exclusive to the Dietzia genus (4).
Figure 2 –Bioinformatics analysis of MftG.(A) Structural model of MftG from M. Smegmatis retrieved from the Alphafold database (26) with the FAD prosthetic group (yellow) modeled into the structure. Green: Rossman fold motif (GxGxxG), red: active site histidine (His411). (B) Collapsed phylogenetic tree (maximum likelihood) of GMC enzymes showing major MftG subfamilies. FastTree support values are shown on branches. The full tree is provided as Supplementary Figure S1 (C) Venn diagram representing the frequency of co-occurrence of mftC (left-medium blue) and mftG (right-dark blue) genes in 312 organisms that encode the MFT gene locus or MftG-like proteins.
To further investigate the co-occurrence of mftG with the mft gene cluster we retrieved a set of genomes encoding MftG or MftC homologs, the latter serving as a proxy for the mft biosynthetic gene cluster (Supplementary Table S1). To refine the annotation of MftGs, we first performed a phylogenetic analysis. To the MftG candidates, we added further GMC-superfamily enzymes from the same genome set as well as sixteen experimentally characterized GMC enzymes used in a previous study (25). Maximum Likelihood analysis (Figure 2B and Supplementary Figure S1) clustered the MftG candidates described by TIGR03970 into a well-supported clade, which comprised MftG proteins from Rhodococcus, other Nocardiaceae and Mycobacteriaceae. The Gordonia and Dietzia MftG candidates were also phylogenetically related to the main MftG clade. We therefore defined all sequences belonging to this clade as MftG. Two sequences previously annotated as MftG, however, were placed outside of the proposed MftG clade. Their corresponding genomes (Nocardia terpenica and Peterkaempfera bronchialis) did not contain any MftC candidate and the MftG candidates were therefore treated as misannotations. We conclude that all MftG candidates are monophyletic. To further improve the annotations, the gene neighborhood of the MftG candidates was investigated, confirming that MftG candidates are frequently encoded within a 5 kb distance from mft genes (Supplementary Table S1). After refinement of the MftG annotation, we proceeded with co-occurrence analysis of MftC and MftG in our microbial genome set. In a total of 312 genomes that contained either MftC or MftG, 311 encoded an MftC homolog, and 235 encoded a putative MftG homolog. In 234 genomes the two genes co-occurred (Figure 2C). This result reinforced the hypothesis that MftG enzymes strictly require mycofactocin. On the other hand, about 25% of the genomes encoding MftC, lack an MftG enzyme. It remains an open question for future investigations, whether other enzymes can complement the function of MftG or whether the function of MftG is dispensable in these organisms.
The role of MftG in growth and metabolism of mycobacteriaTo investigate the physiological role of MftG, a mftG deletion mutant (ΔmftG) was generated in M. Smegmatis MC2 155. Since mft mutant strains typically display defects in ethanol utilization (5), we compared the growth of M. Smegmatis MC2 155 ΔmftG with the WT (wild-type) strain on media containing 10 g L−1 of ethanol as the sole carbon source (Figure 3A). Indeed, almost no growth of ΔmftG was detected on ethanol (Figure 3A), whereas the growth curve of the ΔmftG mutant on glucose-containing media was indistinguishable from the isogenic WT strain (Figure 3B).
Figure 3 –Effect of mftG gene deletion on mycobacterial ethanol metabolism.(A) Growth curve of M. Smegmatis WT, ΔmftG, ΔmftG-mftG, and WT-mftG growing in HdB-Tyl with 10 g L−1 ethanol as the sole carbon source. (B) Growth curve of WT and ΔmftG growing on HdB-Tyl with 10 g L−1 glucose as the sole carbon source. (C, E) Ethanol and acetic acid quantification over time in M. Smegmatis WT, ΔmftG, ΔmftG-mftG, and WT-mftG cultures in HdB-tyloxapol with 10 g L−1 of ethanol and in uninoculated media as control. (D) Growth curve of WT and ΔmftG on HdB-Tyl with 10 g L−1 glucose and 10 g L−1 ethanol combined. (F) Acetaldehyde quantification in culture supernatants of the WT and ΔmftG strains grown on HdB-Tyl with 10 g L−1 glucose and/or 10 g L−1 ethanol. (●) M. Smegmatis WT; () M. Smegmatis ΔmftG mutant; (-dashed) M. Smegmatis ΔmftG mutant grown with starting OD600 1; () M. Smegmatis ΔmftG-mftG complementation mutant; (♦) M. Smegmatis double presence of the mftG gene; () Medium HdB-Tyl with 10 g L−1 of ethanol without bacterial inoculation. Measurements were performed in biological replicates, (growth curves: n≥3, ethanol and acetate quantification: n=3). Error bars represent standard deviations. Statistical analysis was performed with ordinary one-way ANOVA with Tukey's multiple comparison test, p-values depicted in the figure.
We also investigated the growth of the WT and the ΔmftG strain on several related carbon sources and recorded growth curves (Supplementary Figure S2). The growth of WT and mutant strains was not supported by 10 g L−1 methanol, 5 g L−1 hexanol, 0.01 g L−1 acetaldehyde as sole carbon sources as previously reported (5). Notably, WT and ΔmftG cells displayed significant growth with 5 g L−1 acetate or 10 g L−1 glycerol. However, evaluation of the growth using other alcohols showed differential behavior. A prolonged lag phase of the ΔmftG strain was detected on 1-propanol (29 h), 1-butanol (18 h), and 1,3-propanediol (20 h) compared to the WT strain. Interestingly, a putative 1,3-propanediol dehydrogenase (MSMEG_6239) is present in the same operon as MSMEG_6242, indicating that 1,3-propanediol degradation might be also MFT-dependent (27). We further assessed the growth of ΔmftG using a panel of phenotype microarrays. While no further defect regarding carbon source utilization was detected, the ΔmftG mutant showed the ability to grow to a small extent on formic acid in contrast to the WT. Besides the different usage of carbon sources, only minor differences in bacterial growth were found in some of the sensitivity plates (Supplementary Figure S3) demonstrating that the lack of the mftG gene did not induce relevant sensitivity towards antibiotics and stressors compared to WT when grown on glucose alone.
The role of ΔmftG in ethanol metabolism of mycobacteriaSince the utilization of ethanol was the process that was most strikingly impacted by mftG inactivation, it was investigated further. First, the complementation of the ΔmftG deletion using an integrative vector carrying the mftG gene (ΔmftG attB::pMCpAINT-mftG, here named ΔmftG-mftG) was performed and resulted in the restoration of growth on 10 g L−1 ethanol. Intriguingly, the growth curve of the complemented strain, which could be dysregulated in mftG expression, displayed a shorter lag phase, a faster second exponential growth phase, and a higher final biomass yield compared to the WT (Figure 3A). Altered growth kinetics of complement mutants on ethanol are a known phenomenon, albeit not always well understood (28). However, duplication of the mftG gene using the same vector in the WT strain (WT attB::pMCpAINT-mftG, here named WT-mftG) also showed enhanced growth. These results indicate that MftG might catalyze a rate-limiting step during ethanol utilization.
To further characterize the metabolic processes during ethanol consumption, the amount of ethanol and acetic acid present in the growth media with and without mycobacterial strains (WT, ΔmftG, ΔmftG-mftG and WT-mftG) was measured (Figure 3C, E). As reported previously, ethanol consumption during exponential growth of WT mycobacteria was accompanied by acetic acid production (Figure 2E), a process known as overflow metabolism where the rate of ethanol oxidation to acetate exceeds the rate of carbon assimilation for biomass formation during fast growth (8, 29). Although the ΔmftG strain could achieve residual growth after 45 h, the consumption of ethanol and the production of acetic acid remained at a very low level. (Figure 2C). While this finding is in line with the occurrence of a metabolic roadblock in the ΔmftG mutant, it indicates, however, that enzymatic activities supporting ethanol oxidation to acetate are not completely abolished in ΔmftG mutants. This interpretation is also supported by growth curves recorded on a combination of ethanol and glucose, where a simultaneous feeding enhanced the growth yield of the ΔmftG mutant (Figure 2D) suggesting at least partially intact ethanol utilization in ΔmftG cells.
Typically, ethanol is oxidized to acetaldehyde first, which is further oxidized to acetic acid. To test whether the metabolic roadblock affects acetaldehyde formation or consumption, we also quantified acetaldehyde from supernatants of WT and ΔmftG cells grown on ethanol or ethanol combined with glucose (Figure 3F). Interestingly, when comparing WT and the ΔmftG mutant, we noticed that both strains produced a similar amount of acetaldehyde regardless of whether ethanol was used as the sole carbon source or supplemented (Figure 3F), indicating that acetaldehyde formation is not altered in the mutant strain. At this point it may be helpful to revisit the fact that ΔmftG mutants grew well on acetate (Supplementary Figure S2). This observation together with the detection of acetaldehyde and acetate in the supernatants of ΔmftG cultures excludes the hypothesis that mftG is required for acetaldehyde and acetate assimilation. When investigating the carbon metabolism of the ΔmftG-mftG (complement) and WT-mftG (overexpression) strains, an inverted phenotype became visible. Parallel to the accelerated and enhanced growth described above (Figure 3A), the overexpression strains displayed higher rates of ethanol consumption as well as an earlier onset of acetate overflow metabolism and acetate consumption (Figure 3D). These results indicate that MftG activity is, at least indirectly, related to ethanol oxidation. Notably, the accelerated turnover of the volatile substrate ethanol, which is subject to substantial evaporation during the cultivation process, to acetate, which is less volatile, could explain the enhanced final growth yield of the complement and overexpression strains.
The impact of ethanol on survival and cell division of ΔmftG mutantsTo investigate whether the reduced growth of ΔmftG mutants on ethanol is due to limited carbon and energy supply or rather a consequence of insufficient ethanol detoxification, we cultivated bacteria on combinations of glucose and ethanol and recorded growth curves. Surprisingly, simultaneous feeding with 10 g L−1 glucose and 10 g L−1 ethanol even promoted the growth of the ΔmftG mutant. In addition, we determined the percentage of dead cells upon cultivation with different carbon sources using propidium iodide staining followed by flow cytometry quantification (Figure 4A). Regardless of the genotype, the bacterial cultures grown on glucose or ethanol as the sole carbon source and under starvation displayed a similar percentage of dead cells after a cultivation period of 72 h. We, therefore, conclude that the inability of ΔmftG cells to grow on ethanol alone is not due to ethanol toxicity. This conclusion was further supported by measurements of the transmembrane potential that did not reveal any disturbance of the proton motif force (PMF) in the mutant (Figure 4B). Previous studies of the transmembrane potential of M. Smegmatis ΔmftC cells, another mutant unable to grow on ethanol as a carbon source, showed the same pattern with no differences compared to WT (5). However, unexpectedly, an elevated proportion of dead cells was detected when ΔmftG was cultivated on combined carbon sources, while the WT tolerated this condition well. This finding emphasizes the role of the mycofactocin system in enhancing the metabolic adaptability of mycobacterial cells under increasingly complex environmental conditions.
Figure 4 –Phenotypic characterization of mycobacterial strains grown on HdB-Tyl with glucose and/or ethanol or starvation.(A) Quantification of dead cells by flow cytometry using propidium iodide of M. Smegmatis strains grown throughout 72 h. Biological replicates, starvation cells n=2, other conditions n=3. (B) Quantification of cells with normal transmembrane potential by flow cytometry of the M. Smegmatis cultures throughout 72 h. Biological replicates n=3. (C) Super-resolution microscopy images of M. Smegmatis strains at exponential phase or 48h of starvation, labeled with NADA (green), RADA (red), superposition of NADA and RADA (yellow). Bar size: 3 µm. (D) Cell size distribution obtained from super-resolution microscopy of the M. Smegmatis strains at exponential phase or 48h of starvation. (E) Ratio of the number of replication sites to the number of cells of the M. Smegmatis strains cultures at exponential phase or 48h of starvation, together with a microscopy image of a single ΔmftG cell at 48h grown on ethanol, with arrows pointing to the several septa stained with NADA (green) and RADA (red). Bar size: 3 µm.
Color legend: (A,B): ● – sample at 24h; ▪– sample at 48h; ▴– 72h. (C) (D,E): orange – 10 g L−1 glucose; green – 10 g L−1 glucose and 10 g L−1 ethanol; blue – 10 g L−1 ethanol; white – starvation for 48h. Statistical analysis was performed for PI, cell size and ratio of replication sites per cell with ordinary one-way ANOVA, for transmembrane potential with ordinary two-way ANOVA, all using Tukey's multiple comparison test. The p-values are depicted on the figure, microscopy-based analysis performed with technical replicates (n=3).
To reveal potential morphological changes induced by ethanol treatment, we further examined the ΔmftG mutant incubated with different carbon sources by super-resolution microscopy. The identification of elongation and replication sites was achieved by the sequential incubation of the cells with the fluorescent D-amino acid labeling probes NADA (green) and RADA (red) (Figure 4C-E). These probes are incorporated via extracellular routes of mycobacterial peptidoglycan assembly, labeling both elongation poles as well as the sidewall (30). The M. Smegmatis WT and ΔmftG-mftG cultivated on glucose as the sole carbon source appear elongated with mainly polar growth (accumulation of RADA at the poles indicates the most recent active site of peptidoglycan incorporation). NADA occurs mainly at a non-polar location (previous active replication site). The ΔmftG mutant grown on glucose, however, showed few yellow cells (overlap of green and red) indicating growth arrest on a small part of the culture. Some cells are not actively synthesizing peptidoglycan and thus not growing, which leads to the overlap of both dyes (Figure 4C).
Mycobacteria grown on glucose and ethanol showed a regular dispersion profile of both dyes, with polar RADA, non-polar NADA, and occasional mid-cell septum formation with no obvious differences between the three genotypes. In contrast, cultivation on 10 g L−1 ethanol alone as well as under starvation condition (no carbon source) had a strong influence on peptidoglycan synthesis as the dyes show decreased incorporation into the cell wall. The simultaneous incorporation of both dyes in WT and ΔmftG-mftG cells grown on ethanol as the sole carbon source reflected the reduced growth rate, as shown by the growth curves (Figure 3A, B) compared to the glucose condition. Interestingly, ΔmftG mutant cells struggled to divide on ethanol displaying multiple septa and extreme elongation compared to WT on ethanol, leading to a higher ratio of foci per cell compared to WT grown on the same condition (Figure 4D, E). Independent of the genotype, the cultivation in the presence of ethanol significantly decreased cell size. A similar phenotype was previously observed in Mycobacterium vaccae cells exposed to ethanol (31). Starving cells were also significantly reduced in size in WT and ΔmftG strains compared to growth on glucose alone (Figure 4C, E), reflecting the limitation of resources available for propagation and elongation. Especially under starvation conditions, the cells appeared predominantly yellow, suggestive of growth arrest. A previous study on the effect of starvation on mycobacteria also showed the formation of large cells with multiple septa as a hallmark of starvation, from which after 14 days, mildly starved cells (with traces of carbon source available) remodeled into small resting cells (32). Our results indicated that the major phenotype of ΔmftG on ethanol as a sole source of carbon is a growth defect comparable to a starvation effect. When incubated solely with this carbon source, ΔmftG cells are almost unable to divide, but remain alive and in a metabolically active state, most likely consuming ethanol in trace amounts.
Impact of mftG on mycobacterial cofactor metabolismBased on the results above, we concluded that MftG is crucial for proper ethanol metabolism in mycobacteria. However, deletion of its corresponding gene still allowed for basal metabolic activity and ethanol oxidation. One way to explain these findings is that MftG is involved in cofactor regeneration during growth on ethanol as a carbon source. The genetic linkage of mftG with mycofactocin biosynthesis supported the hypothesis that MftG might be responsible for mycofactocin regeneration. However, before directly addressing mycofactocin metabolism, we decided to monitor the central respiratory redox cofactor NAD. We therefore determined the NADH/NAD+ ratios of WT and KO during the exponential phase when bacteria were grown on glucose or ethanol as the sole carbon sources. However, the presence or absence of mftG did not significantly influence the NADH/NAD+ ratio of bacteria grown on either carbon source (Figure 5A). This finding indicated that NAD homeostasis remained intact in the ΔmftG mutant. Notably, when looking at absolute NAD+ levels, (Figure 5B) an increase of the NAD pool in cells growing on ethanol was detected, however, independent of the genotype. Along with central redox (NAD+) metabolism, we also monitored the central energy metabolism of the cells by determining ADP/ATP ratios. These clearly supported the hypothesis that ΔmftG cells suffered from starvation. The mutant strain exhibited a significant energy deficit at all three sampled time points, with the energy depletion progressively worsening between 24 and 48 hours of incubation (Figure 5C). Previous studies of M. Smegmatis under starvation also showed a reduction of ATP levels as a result of hampered energy metabolism (32). It should be mentioned that ATP synthesis strictly depends on respiration in M. Smegmatis. Mycobacteria typically cannot sustain growth, even on fermentable substrates, via substrate-level phosphorylation alone (33). After confirming energy shortage but intact NAD homeostasis in ΔmftG cells, we decided to test whether MftG is involved in mycofactocin regeneration. To this end, we directly analyzed the MFT pool using targeted liquid chromatography-high resolution mass spectrometry (LC-MS). We have previously shown that the total pool size of MFT species is highly expanded when M. Smegmatis uses ethanol as a sole carbon source and that both reduced (M/MFT-nH2) as well as oxidized species (M/MFT-n) are stable when extracted and can thus be detected by LC-MS (8). Here, we performed a comparative analysis of the metabolome of M. Smegmatis WT and ΔmftG grown in HdB-Tyl supplemented with either 10 g L−1 glucose, 10 g L−1 ethanol, or both carbon sources combined (10 g L−1 glucose and 20 g L−1 ethanol). Metabolites were extracted during the exponential or stationary growth phase. Additionally, metabolome extracts from complemented ΔmftG-mftG and WT-mftG strains grown on HdB-Tyl with 10 g L−1 ethanol sampled only at the stationary phase were analyzed. Strikingly, this analysis revealed significantly elevated levels of MMFT-8H2 in ΔmftG compared to the other strains tested (Figure 5D-F). MMFT-8H2 is the major representative of reduced mycofactocins (mycofactocinols) in M. Smegmatis. Interestingly, ΔmftG strains contained almost none of the corresponding oxidized mycofactocinone (MMFT-8) in any of the conditions tested. These results strongly supported the hypothesis that MFT regeneration was hampered in the ΔmftG mutant while MFT reduction was still taking place, thus resulting in a near-total conversion of mycofactocinones to mycofactocinoles. This phenotype was also observed when ΔmftG cells were cultivated on glucose and ethanol combined (Figure 5E) and even on glucose alone (Figure 5D). Complementation of the ΔmftG mutant (ΔmftG-mftG) reverted the MMFTH2 to MMFT ratio back to WT levels. In contrast, the overexpression strain WT-mftG grown in ethanol contained significantly more MMFT-8 (oxidized) compared to other strains in the same condition (Figure 5F), thus providing further evidence that MftG is involved in reoxidation of mycofactocin and re-enforcing that this step might even represent a rate-limiting step during ethanol utilization.
Figure 5 –Cofactor metabolism of M. Smegmatis strains.(A) NADH/NAD+ ratio of M. Smegmatis WT and ΔmftG grown on HdB-Tyl with either 10 g L−1 glucose or 10 g L−1 ethanol at exponential phase. (B) NADH and NAD+ quantification of M. Smegmatis WT and ΔmftG grown on HdB-Tyl with either 10 g L−1 glucose or 10 g L−1 ethanol at exponential phase. (C) ADP/ATP ratio of M. Smegmatis WT and ΔmftG grown on HdB-Tyl with 10 g L−1 ethanol at 24 h, 48 h and 72 h. (D, E, F) Targeted comparative metabolomics of M. Smegmatis WT, ΔmftG, ΔmftG-mftG, and WT-mftG strains. The most representative MFT species, methylmycofactocinone with 8 glucose moieties (MMFT-8H2, sum formula: C62H99NO43, RT: 6.82 min, m/z 1546.5665 [M+H]+) and methylmycofactocinol with 8 glucose moieties (MMFT-8, sum formula: C62H97NO43, RT: 7.18 min, m/z 1544.5507 [M+H]+), was used to reflect MFT obtained from M. Smegmatis stra

Comments
Post a Comment