Humans Inoculated with Genetically Modified Malaria Parasites - The Scientist
Two clinical trials, in which subjects were vaccinated with genetically engineered Plasmodium parasites and later exposed to the malaria-causing microbe, showed the vaccines to be safe with promising, but not ideal, efficacy. Results of the trials are published in two papers in Science Translational Medicine today (May 20).
“Within the malaria control and elimination space, we do need additional tools, so . . . these are very welcome results,” says malaria and public health expert James Tibenderana of the Malaria Consortium, a nonprofit organization that carries out research, develops policies, and advocates for the prevention, control, and treatment of malaria. Tibenderana, who was not involved in either study, adds, “They are cutting-edge innovations to attempt to customize a vaccine.”
Certain parasites of the Plasmodium genus, including P. falciparum and P. vivax, cause malaria when transmitted to humans via mosquito bites. These parasites have “impacted human health throughout history,” says malaria researcher Stefan Kappe of the University of Washington and Seattle Children’s Research Institute who is developing his own engineered parasite vaccine but was not involved in the studies. With more than 200 million cases of malaria causing more than 400,000 deaths per year, the majority of which are children, “we urgently need a vaccine,” Kappe adds.
Developing one has been a decades-long challenge, and while candidates exist, none are ideal, says Miguel Prudêncio of the Institute of Molecular Medicine in Portugal, author of one of the papers. The vaccine furthest along in clinical development is RTS,S, which is composed of a viral vector carrying a highly immunogenic Plasmodium antigen called circumsporozoite protein (CSP). A pilot study of RTS,S is currently underway in Malawi, but the vaccine protects only about 40 percent of recipients and has some safety concerns.
An alternative vaccination approach is to use the whole attenuated parasite, Prudêncio says, the idea being that the live but weakened bug will activate the immune system more potently than non-living protein vaccines. One strategy for attenuation is to blast the parasites with radiation. Injection of irradiated P. falciparum has been shown to protect more than 80 percent of recipients from subsequent challenge with infectious parasites. But, because irradiation doesn’t affect each parasite equally—some are mildly affected, others killed—and because dead parasites “probably don’t contribute” as much to immunization, “it is a very inefficient production process,” says Robert Sauerwein of Radboud University in the Netherlands who coauthored both papers. “You need many, many parasites and that means many, many mosquitoes” to get a dose of vaccine.
Sauerwein and colleagues have instead developed genetically attenuated parasites that should be “cheaper and safer,” he says. Cheaper because all the parasites are identical so fewer should be needed to elicit the same effect as that seen with irradiated bugs. And safer because there is practically no chance of the parasites reverting to infectious forms (a slim but not zero chance in the case of irradiation), and there is no need for handling the infectious form whatsoever. The rearing of thousands of mosquitoes carrying disease-causing P. falciparum is necessary in the case of irradiation, but Sauerwein’s mosquitoes carry only the engineered, and therefore safe, version.
Sauerwein’s engineered P. falciparum is missing two genes essential for the parasite’s maturation in the human liver—the first stage of infection following a mosquito bite. This prevents its subsequent infection of red blood cells—the second stage of infection in which the parasite replicates wildly and the disease becomes symptomatic.
In the trial, 25 volunteers were injected with the modified parasites three times, at eight-week intervals. The vaccinations were well tolerated and the subjects developed anti-Plasmodium antibodies. They also had increased resistance to challenge with wildtype P. falciparum. Three of the 25 vaccine recipients were protected from disease while the remaining 22 showed a significant delay in parasite detection in the blood compared with unvaccinated controls, indicating the vaccine-induced immunity helped slow disease progression. Once parasites were detected in a participant’s blood, they were given anti-malarial drugs to stop the symptoms. A group of 13 volunteers vaccinated with irradiated P. falciparum as a positive control all showed delayed parasite detection, but none were protected. Why the positive control failed to protect to the degree previously reported (approximately 50 percent protection at the dose given, or 80–90 percent had it been a full dose) is unclear, says Sauerwein.
With the positive control and the test vaccines performing more poorly than anticipated, “we could have been happier,” says Sauerwein, “but we’re moderately happy with the results.”
“They’ve shown that the organism is fully attenuated by their particular gene deletion and that it is safe in humans so far, and can produce low levels of protective immunity . . . but is still not quite optimal,” says Kappe.
In the second paper, Sauerwein, in collaboration with Prudêncio and colleagues, report the results of a trial with a different genetically modified Plasmodium. Instead of attenuating P. falciparum, the team used a strain that infects mice but is naturally harmless to humans—P. berghei—and introduced into its genome the code for P. falciparum’s CSP protein.
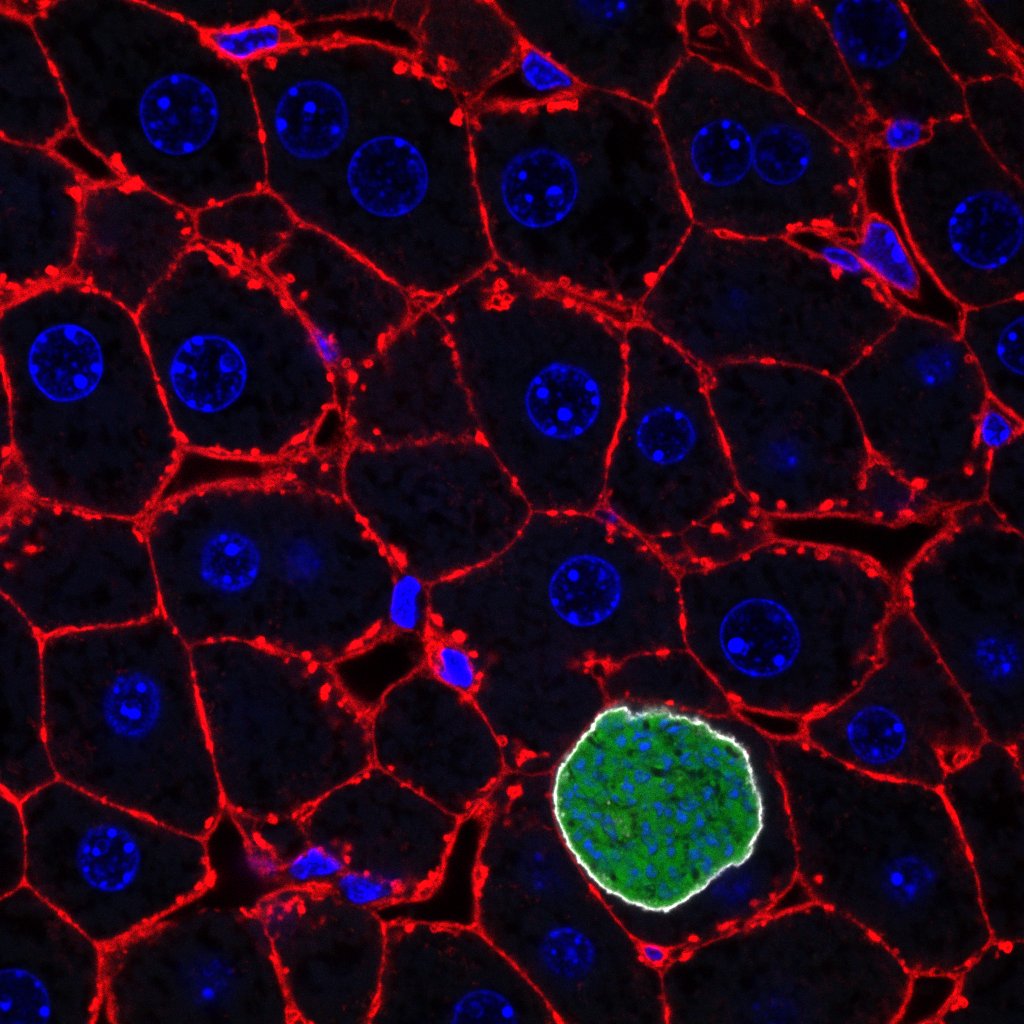
Plasmodia (green) growing in liver cells
ANTONIO MENDES
While Sauerwein collaborated with Sanaria Inc—a biotech company with technology for creating injectable parasite vaccines—in the P. falciparum study, the company was not involved in the P. berghei study. So, twenty-four volunteers were inoculated the old fashioned way, with 75 bites from mosquitoes infected with the modified parasite. The dose was repeated a further three times for a total of 300 bites. “Believe it or not there were no drop outs,” says Prudêncio, explaining that all the volunteers braved the itching through to the end of the trial.
Again, the vaccinations were well tolerated and induced anti-CSP antibodies. While none of the subjects were fully protected from subsequent infection with wildtype P. falciparum, the vaccinations did significantly delay the detection of live parasites in the blood, says Prudêncio. He says he thinks that increasing the dose of the vaccine (via injection of parasites in collaboration with Sanaria, rather than more bites) and tweaking the parasite to enhance immunogenicity, are likely to improve the vaccine’s performance.
These trials are proof-of-principal studies and genetically engineered parasites “are still a long way away” from prime time, says Tibenderana. Nevertheless, the results are “important,” he adds.
“I think we’re seeing the light at the end of the tunnel with malaria vaccines,” says Kappe. “Overall, it’s super promising, super exciting.”
M. Roestenberg et al., “A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1,” Sci Transl Med, 12:eaaz5629, 2020.
I.J. Reuling et al., “An open-label phase 1/2a trial of a genetically modified rodent malaria parasite for immunization against Plasmodium falciparum malaria,” Sci Transl Med, 12:eaay2578, 2020.

Comments
Post a Comment